HbA1c variability may have an association with increased cardiovascular and CKD events
CONTINUING EDUCATION
To earn CEUs, visit www.mlo-online.com under the CE Tests tab.LEARNING OBJECTIVES
1. Discuss diabetes mellitus (DM) in terms of health outcomes, complications, and laboratory testing.
2. Define limitations of current studies regarding DM patients and HbA1c variability.
3. Discuss the design, population, and variables of the study described in the article.
4. Identify key conclusions of the study and additional findings from further studies.
The number of people with diabetes mellitus (DM) is growing worldwide. People with diabetes have a two- to three-fold increased risk of developing cardiovascular disease, which remains the major cause of death in such patients.1,2 Several studies have demonstrated the importance of time-averaged mean levels of glycemia, as measured by HbA1c, which has since been considered on of the best methods available for measuring glycemic control.3,4 However, recent research has pointed out that plasma glucose variability, regardless of hyperglycemia level, may confer additional risk for the development of micro- and macrovascular diabetic complications.5-7
Among studies on type 2 DM with preserved renal function, glucose variability is positively associated with the development of diabetic retinopathy, neuropathy, and nephropathy,8-11 but its effects on macrovascular events remain uncertain. Wan et al. evaluated the association of HbA1c variability with cardiovascular events in patients with type 2 DM and found that high variability was associated with increased cardiovascular events.12 On the other hand, the cross-sectional analysis within the Italian Renal Insufficiency and Cardiovascular Events (RIACE) multi-center study showed no impact of HbA1c variability.11 Thus, current literature is conflicting and limited, especially on different renal functions.
Even less is known about how glycemic control affects prognosis in diabetic patients with late stage chronic kidney disease (CKD), especially since these patients are often excluded from clinical trials. Few studies have evaluated the association of HbA1c variability with cardiovascular events in diabetic patients with moderate-to-advanced CKD. This study therefore aimed to assess whether HbA1c variability is associated with cardiovascular events in type 2 DM patients. It also tested whether different renal functions affect such an association.
The study’s ground rules
Setting: This study used data from the Kaohsiung Medical University Hospital Research Database (KMUHRD). Kaohsiung Medical University Hospital (KMUH), established in 1957, is a medical center with 1,600 beds, 1,200 general beds, 144 intensive care beds, and about 6,000 clinical visits per day in 2015. The KMUHRD includes data for approximately two million patients who attended KMUH in the period 2009–2015, predominantly from southern Taiwan.
The KMUHRD offers a comprehensive database with coverage on ambulatory care, hospital admissions, dental services, drug-dispensing records, and biochemical data. The hospital records include detailed information on primary and secondary diagnoses, procedures, and dates of hospital admission and discharge. All diagnoses are coded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The drug-dispensing histories contain data on the dispensed drug, prescriber type, dispensing date, dispensed amount, prescribed dose regimens, and duration of use (prescription length).
The database is managed by the KMUH Division of Medical Statistics and Bioinformatics. For confidentiality and in accordance with the Personal Information Protection Act, all personal identifiers were removed and the authorized researchers only performed data linkage, processing, and statistical analyses with specified computers in an independent 24-hour monitored room using encrypted identifiers. All data analysts were required to sign an agreement. Only tables and figures from statistical analysis were allowed to be released after inspection.
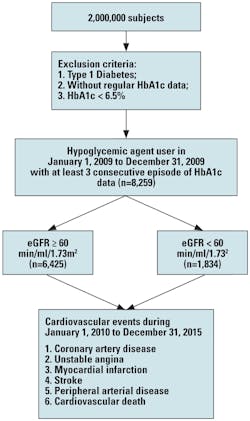
Study population: The entry date was between January 1, 2009, and December 31, 2009. All patients with type 2 diabetes on hypoglycemic agents and with an HbA1c level of ≥ 6.5 percent were enrolled and followed-up from January 1, 2010, until December 31, 2015. Data on the occurrence of cardiovascular events, including coronary artery disease, myocardial infarction, ischemic stroke, and peripheral arterial disease on follow-up were also obtained from the KMUHRD. The exclusion criteria were type 1 diabetes, mortality, DM diagnosis after a cardiovascular event, use of insulin-dependent and -independent agents, and HbA1c < 6.5 percent. The study patients were then stratified into two groups based on their estimated glomerular filtration rate (eGFR) ≥ 60 and < 60 min/ml/1.73m2 (Figure 1). We classified our patients with evidence of kidney damage lasting for more than three months into CKD stage 3, 4, and 5, based on estimated eGFR level (mL/min/1.73 m2) of 30 to 59, 15 to 29, and < 15, respectively.
The data recorded before entry into the study were age, sex, duration of diabetes, hypertension, hyperlipidemia, nephropathy, retinopathy, and neuropathy. Urine albumin and creatinine were measured on a spot urine sample by an autoanalyzer (COBAS Integra 400 plus; Roche Diagnostics), and nephropathy was defined as the ratio of urine albumin to creatinine of ≥ 30 mg/g.
Definition of study endpoint: The study endpoint was defined as cardiovascular events, including coronary artery disease, stroke, peripheral arterial disease, and cardiovascular death. Coronary artery disease was defined as a history of angina and ischemic electrocardiogram change, ST-elevation myocardial infarction, non ST-elevation myocardial infarction, unstable angina, or having undergone coronary bypass surgery or angioplasty.
HbA1c variability: The intra-personal mean and standard deviation (SD) of HbA1c was calculated for each patient, and the latter was considered an index of HbA1c variability. The estimated HbA1c variability was based on at least three HbA1c measurements.
Statistical analysis: All statistical analyses were performed using the SPSS 19.0 for Windows (SPSS Inc. Chicago, IL). Data were expressed as percentages or mean ± standard deviation. Between-group differences were assessed using the chi-square test for categorical variables and the independent t-test for continuous variables. The association between HbA1c variability and cardiovascular events was assessed using multivariate Cox proportional hazard analysis. Age, sex, and significant variables in the univariate analysis were used in the multivariate analysis. The Kaplan-Meier analysis was used to determine the survival curve for cardiovascular events. Statistical significance was set at p<0.05.
The study’s results
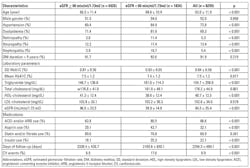
Comparisons of baseline data between patients with eGFR ≥ and those with < 60 min/ml/1.73m2 were shown (Table 1). The patients’ mean age was 62.0 ± 11.9 years. Compared to patients with eGFR ≥ 60 min/ml/1.73m2, patients with eGFR < 60 min/ml/1.73m2 were older and had higher prevalence rates of hypertension, dyslipidemia, diabetic retinopathy, neuropathy, and nephropathy. They had higher HbA1c SD, triglyceride, and total cholesterol levels, but lower mean HbA1c, high-density lipoprotein (HDL) cholesterol, and eGFR. They also had higher percentage use of angiotensin converting enzyme inhibitors (ACEIs) and/or angiotensin II receptor blockers (ARBs), aspirin, and insulin. In addition, patients with eGFR < 60 min/ml/1.73m2 had fewer follow-up days and a higher frequency of cardiovascular events (p < 0.001).
In a mean follow-up period of 6.3 years, cardiovascular events were recorded in 8.9 percent of the patients. These included hospitalization for coronary artery disease (n = 876), stroke (n = 100), peripheral artery disease (n = 54), and cardiovascular death (n = 27).
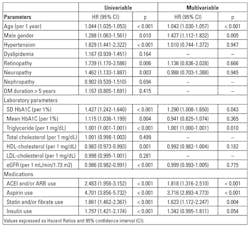
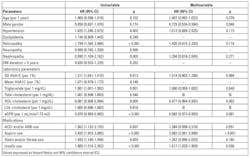
Risk of cardiovascular events in patients with eGFR ≥ 60 min/ml/1.73m2: Cox proportional hazard regression analysis was used to evaluate the association between SD of HbA1c and cardiovascular events in patients with eGFR ≥ 60 min/ml/1.73m2 (Table 2). Old age, male sex, hypertension, diabetic retinopathy and neuropathy, high HbA1c SD, high mean HbA1c, high triglyceride, low HDL-cholesterol, low eGFR, ACEI and/or ARB use, aspirin use, statin and/or fibrate use, and insulin use were associated with significantly higher risk of cardiovascular events.
By multivariate analysis, old age, male sex, high HbA1c SD, high triglyceride, ACEI and/or ARB use, aspirin use, and statin and/or fibrate use were associated with significantly increased risk of cardiovascular events.
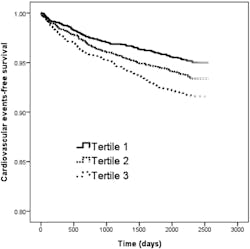
The Kaplan-Meier curves for cardiovascular event-free survival of study patients with eGFR ≥ 60 min/ml/1.73m2 according to tertiles of SD of HbA1c (<0.48 and ≥ 0.48 to < 0.90 and ≥ 0.90 percent) revealed that tertile 2 and tertile 3 patients had worse cardiovascular events-free survival than those in tertile 1 (Figure 2).
Diabetic patients with tertile 2 and tertile 3 of HbA1c SD had a worse cardiovascular events-free survival than those with tertile 1 of HbA1c SD.
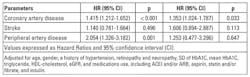
We further performed the analysis of relation of SD of HbA1c for separate cardiovascular events in patients with eGFR <= 60 min/ml/1.73m2, and found that high HbA1c SD was still associated with significantly increased risk of coronary artery disease after multivariate analysis (Table 3).
Risk of cardiovascular events in patients with eGFR < 60 min/ml/1.73m2. The Cox proportional hazard regression was used to evaluate the association between SD of HbA1c and cardiovascular events in patients with eGFR < 60 min/ml/1.73m2 (Table 4). By univariate regression analysis, old age, female sex, hypertension, diabetic retinopathy and nephropathy, high HbA1c SD, high triglyceride, low HDL-cholesterol, low eGFR, ACEI and/or ARB use, aspirin use, statin and/or fibrate use, and insulin use were associated with significantly greater risk of cardiovascular events.
In the multivariate analysis, female sex, low eGFR, aspirin use, and insulin use were associated with significantly higher risk of cardiovascular events. However, HbA1c SD was not.
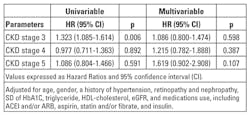
We further performed the analysis of relation of SD of HbA1c for cardiovascular events in patients with different CKD stage, and found that no significant association was noted in each CKD stage after multivariate analysis (Table 5).
The clinical significance of the findings
This study investigated the association between HbA1c variability and cardiovascular events in patients with type 2 DM over a follow-up period of 6.3 years. The results show that patients with greater HbA1c variability have increased risk of cardiovascular events in diabetic patients with eGFR ≥ 60 min/ml/1.73m2, but not in those with eGFR < 60 min/ml/1.73m2.
The first important finding here is that among diabetic patients with higher eGFR, greater HbA1c variability is associated with increased risk for cardiovascular events, independent of mean HbA1c levels. In a subsequent post hoc analysis, the Diabetes Control and Complications Trial (DCCT) data investigated the relationship between the risk of progression of retinopathy and HbA1c levels after five to nine years of follow-up.13 The results revealed that the patients who received intensive treatment had a significantly lower risk of the progression of retinopathy than those with conventional treatment despite comparable HbA1c levels. The authors concluded that the mean level of HbA1c may not be the key factor determining the degree of hyperglycemia, and that other risk factors independent of HbA1c level may increase the risk of complications from diabetes due to additional metabolic burden.13 Such findings may be partly due to the fact that studies have used HbA1c as a marker of glucose control, which fails to reflect glucose variability and the risk associated with extreme glucose change over an extended time period.
The association between HbA1c variability and the development of cardiovascular events may be due to several mechanisms. In an in vitro study, Risso et al. reported that fluctuations in glycemia have a more harmful effect on endothelial cells than a persistently high level of glucose. They also found that apoptosis (cell death) was significantly higher in human umbilical vein endothelial cells incubated in media in which the glucose concentration was alternately set at 5 or 20 mmol/l every other day for 14 days compared to a fixed high concentration of glucose.14 Furthermore, intermittently high glucose levels enhance the formation of markers of oxidative stress, leading to increased cellular apoptosis.15
In an in vivo study, Horva´th et al. reported that rats intermittently treated with glucose had a significantly worse endothelial function than those that were untreated despite a lower total exposure to glucose. Furthermore, the authors also hypothesized that the poly(ADP-ribose) polymerase pathway had been activated.16 Human studies also showed that acute glucose fluctuations strongly correlated with triggering oxidative stress, urinary levels of 8-iso PGF2α, and more endothelial function damage, leading to higher levels of oxidative stress markers than constantly high glucose.17,18 Some studies reveal that glucose variability is associated with the risk of hypoglycemia,19,20 which, in turn, may contribute to increased progression of cardiovascular disease and mortality by inducing inflammation, blood coagulation abnormality, sympatho-adrenal response, and endothelial dysfunction.21,22
In the present study, the impact of HbA1c variability on the risks of cardiovascular events in diabetic patients with preserved renal function remained significant after adjustments in the mean HbA1c. This suggests that HbA1c variability may provide additional valuable information as a potential predictor of adverse cardiovascular outcomes. Another important finding here is that the significant association between HbA1c variability and cardiovascular events is not observed in patients with moderate-to-advanced CKD. This implies that the predictor power of HbA1c variability on the risk of cardiovascular events among patients with CKD may be relatively lower.
The prognostic role of HbA1c variability in such patients is unclear because of impaired glucose metabolism in CKD, while the HbA1c level may be altered by anemia or by the use of an erythropoiesis-stimulating agent. In patients with CKD, HbA1c formation is reduced because the fragile red blood cell (RBC) has a shortened lifespan by 30 percent to 70 percent and carbamylated hemoglobin molecules in a uremic environment become resistant to glycosylation.23 Young RBCs have a lower glycosylation rate compared to old RBCs, thereby altering HbA1c formation.24 Thus, although aggressive glycemic control may be beneficial for early cardiovascular disease prevention, there is a paucity of data on outcomes supporting tight glycemic control in patients with CKD, and HbA1c variability may be a more useful indicator in preserved renal function for predicting clinical outcomes.25
This study has several limitations. First, as an observational study, the number and frequency of HbA1c measurements varied among individual patients. To minimize this, patients with fewer than three HbA1c measurements during the follow-up period and those with a follow-up period shorter than six months were excluded. Second, we used DM duration > 5 years instead of continuous diabetes duration. The diabetes durations were always underestimated in the case of diabetes due to underdiagnosis. Referred to most of the studies, categorical division was used for DM duration and the diabetes complications were started as early as five years after the diagnosis of diabetes. So we preferred to follow most of the studies reported by using categorical division of <= and > 5 years in diabetes duration.
In addition, post hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD)26 and other trials suggests that cardiac autonomic neuropathy may be a predisposing factor to cardiovascular events. However, the patients here were not evaluated for heart rate variability and, as such, the effect of cardiac autonomic neuropathy on cardiovascular events could not be assessed. Nonetheless, a diagnosis of diabetic neuropathy according to the ICD-9-CM code is included in the analysis.
Last, the effect of medications on cardiovascular events was not evaluated because this study was not a clinical trial aimed at investigating the effects of medication. There is insufficient data on cumulative exposure duration and defined daily dose. In this study, the positive correlation between medications use and cardiovascular events may be due to selection bias.
In conclusion, greater HbA1c variability is associated with increased risk of cardiovascular among patients with eGFR ≥ 60 min/ml/1.73m2, but not in patients with eGFR < 60 min/ml/1.73m2. These findings support the potential role of glucose variability, characterized by SD of HbA1c, as a predictor of cardiovascular events, independent of mean HbA1c level, in patients with type 2 DM with preserved renal function. Clinicians should be cautious about fluctuations along with the direction of changes for preventing the development of adverse cardiovascular outcomes.
Citation: Lee M-Y, Hsiao P-J, Huang Y-T, Huang J-C, Hsu W-H, Chen S-C, et al. (2017) Greater HbA1c variability is associated with increased cardiovascular events in type 2 diabetes patients with preserved renal function, but not in moderate to advanced chronic kidney disease. PLoS ONE 12(6): e0178319.https://doi.org/10.1371/journal.pone.0178319
The manuscript was lightly edited for style.
REFERENCES
- Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. New Engl J Med.2011;364:829–841. pmid:21366474.
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. pmid:20609967.
- Hanefeld M. Postprandial hyperglycaemia: Noxious effects on the vessel wall. Int J Clin Pract.2002;Suppl 129:45–50.
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (ukpds 35): Prospective observational study. BMJ. 2000;321:405–412. pmid:10938048.
- Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications.2005;19:178–181. pmid:15866065.
- Brownlee M, Hirsch IB. Glycemic variability: A hemoglobin a1c-independent risk factor for diabetic complications. JAMA.2006;295:1707–1708. pmid:16609094.
- Hanefeld M, Temelkova-Kurktschiev T. Control of post-prandial hyperglycemia—an essential part of good diabetes treatment and prevention of cardiovascular complications. Nutr Metab Cardiovasc Dis.2002;12:98–107. pmid:12189909.
- Jun JE, Jin SM, Baek J, Oh S, Hur KY, Lee MS, et al. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:70. pmid:26041130.
- Sugawara A, Kawai K, Motohashi S, Saito K, Kodama S, Yachi Y, et al. Hba(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba kawai diabetes registry 2. Diabetologia.2012;55:2128–2131. pmid:22580991.
- Lin CC, Chen CC, Chen FN, Li CI, Liu CS, Lin WY, et al. Risks of diabetic nephropathy with variation in hemoglobin a1c and fasting plasma glucose. Am J Med.2013;126:1017 e1–10.
- Hsu CR, Chen YT, Sheu WH. Glycemic variability and diabetes retinopathy: A missing link. J Diabetes Complications. 2015;29:302–306. pmid:25534877.
- Wan EY, Fung CS, Fong DY, Lam CL. Association of variability in hemoglobin a1c with cardiovascular diseases and mortality in chinese patients with type 2 diabetes mellitus—a retrospective population-based cohort study. J Diabetes Complications. 2016;30:1240–1247. pmid:27318537.
- Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes. 2008;57:995–1001. pmid:18223010.
- Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924–930. pmid:11595647
- Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: The role of protein kinase c and nad(p)h-oxidase activation. Diabetes. 2003;52:2795–2804. pmid:14578299.
- Horvath EM, Benko R, Kiss L, Muranyi M, Pek T, Fekete K, et al. Rapid ‘glycaemic swings’ induce nitrosative stress, activate poly(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia. 2009;52:952–961. pmid:19263033.
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA.2006;295:1681–1687. pmid:16609090.
- Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. pmid:18299315.
- Murata GH, Hoffman RM, Shah JH, Wendel CS, Duckworth WC. A probabilistic model for predicting hypoglycemia in type 2 diabetes mellitus: The Diabetes Outcomes in Veterans Study (DOVES). Arch Intern Med. 2004;164:1445–1450. pmid:15249354.
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the accord, advance, and va diabetes trials: A position statement of the american diabetes association and a scientific statement of the american college of cardiology foundation and the american heart association. J Am Coll Cardiol. 2009;53:298–304. pmid:19147051.
- Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389–1394. pmid:20508232.
- Saisho Y. Glycemic variability and oxidative stress: A link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15:18381–18406. pmid:25314300.
- Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis. 2004;44:715–719. pmid:15384023.
- Uzu T, Hatta T, Deji N, Izumiya T, Ueda H, Miyazawa I, et al. Target for glycemic control in type 2 diabetic patients on hemodialysis: Effects of anemia and erythropoietin injection on hemoglobin a(1c). Ther Apher Dial. 2009;13:89–94. pmid:19379146.
- Menon V, Sarnak MJ. The epidemiology of chronic kidney disease stages 1 to 4 and cardiovascular disease: A high-risk combination. Am J Kidney Dis. 2005;45:223–232. pmid:15696466.
- Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC Jr, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. New Engl J Med.2011;364:818–828. pmid:21366473.
Authors: Mei-Yueh Lee, Pi-Jung Hsiao, Yu-Ting Huang, Jiun-Chi Huang, Wei-Hao Hsu, Szu-Chia Chen, and Shyi–Jang Shin serve in various clinical, research, and teaching capacities at Kaohsiung Medical University, Kaohsiung, Taiwan.