Emerging applications in clinical mass spectrometry
Mass spectrometry (MS) is being used by an increasing number of clinical laboratories to measure small sample volumes with improved confidence across an expanding range of healthcare applications—from toxicology to personalized medicine. Enhanced assay performance and new laboratory-developed test (LDT) methods offer rapid results at a reduced cost and potentially more clinically relevant information, enticing more clinical core testing laboratories to implement mass spectrometry technology.
Current clinical mass spectrometry applications
With origins in basic research, mass spectrometry emerged as a clinical research tool when it was first applied to fingerprint molecules for drug screening in the fight against drugs of abuse. Since then, the technique has evolved, and with the advent of tandem mass spectrometry (MS/MS) technology to support targeted experimental testing—combined with separation technologies such as gas chromatography (GC), liquid chromatography (LC), and ion mobility spectrometry (IMS)—ever smaller concentrations and metabolites can be targeted. Macromolecule ionization methods, such as electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI), enable the study of protein structure.
The adoption of liquid chromatography-tandem mass spectrometry (LC-MS/MS) for small molecule targeting and translational clinical applications was driven by a need for higher immunoassay accuracy, a lack of availability in approved immunoassays, and the desire for cost reduction. While sample preparation can be more labor-intensive than immunoassays and an ever-increasing demand for automation integration, in-house mass spectrometry-based assays can be cost-effective, even for smaller labs.1
MS technology is now routinely applied in clinical laboratories to improve the sensitivity and specificity of clinical tests, screen for diseases, monitor drug therapy, analyze peptides and proteins for diagnostic testing, and identify causes of infections for targeted therapies.2,3
Technology drivers of mass spectrometry
The American Association for Clinical Chemistry (AACC) and Mass Spectrometry and Separation Sciences (MS3) carried out a survey of 63 clinical laboratory representatives to determine the outlook for clinical mass spectrometry testing. Results indicate the current demands that are likely to drive the future technology trends in clinical mass spectrometry.
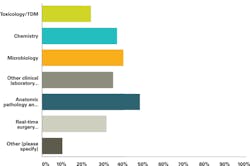
The organizations asked: Of the environments where mass spectrometry tools ARE NOT currently in use, in which ones would you like to see the technology take hold by 2020? The most frequent answers are shown in Figure 1, below. Other uses that were specified by the survey respondents include continued immunoglobulin work, protein analysis, and peptide determinations, and large molecule bioanalysis. To support new clinical mass spectrometry applications, laboratories expect to see improved technical functionality to automate sample acquisition and preparation as well as data analysis and reporting.
SOURCE: AACC / MSSS Outlook for Clinical Mass Spec Testing Q13 p15 4
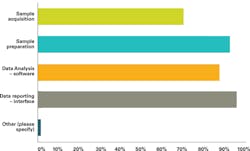
AACC and MS3 also asked: In 2020, which of the following laboratory processes would you anticipate will be automated on your mass spec testing line? The responses are shown in Figure 2.
SOURCE: AACC / MSSS Outlook for Clinical Mass Spec Testing Q9 p10 4
Advancing technologies and potential applications
Recent advances in clinical research and MS technology promise even more sophisticated extensions to current clinical applications and potential new LDTs.
Matrix assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS) now supports direct tissue analysis with diagnostic potential and reduced analysis time.5 Pathologists are now able to examine biological tissue directly, detecting cancerous tissue in real time during an operation using MS with smart electroknives.6 Mass spectrometry imaging (MSI) enables histologists to define tissue types by chemical composition rather than structure.7
Able to distinguish and measure the separate contributions of molecules such as the 25-hydroxy vitamins D2 and D3 and thyroid hormones, MS/MS methods have enabled clinical laboratories to overcome the limitations of immunoassays caused by nonspecific antibody binding and cross-reactivity with metabolites.8 Endocrinologists are also now investigating a “lab on a plate” method to more accurately predict risk of heart attack and stroke in diabetes patients.6 Application of MS technology in clinical and translational research is opening up new lines of discovery in blood-based biomarkers, tumor markers, and even endogenous metabolites as new disease biomarkers.9,10
More sensitive urine screening methods capable of detecting designer drugs are available in the market, and vendor investments assure continued development. Some clinical research and forensic toxicology laboratories already use high-resolution, accurate-mass (HRAM) or time-of-flight (TOF) MS, for multi-analyte drug screens. Whereas triple quad-based techniques can quantitate targeted analytes, newer HRAM systems can simultaneously detect and deliver the full product ion spectrum, and retain high selectivity with greater resolution and mass accuracy.
Advanced mass spectrometry methods are producing novel functional assays. Liquid chromatography MS/MS using online solid-phase extraction (XLC-MS/MS) was used to develop a plasma renin activity (PRA) assay for monitoring mineralocorticoid therapy and screening hypertensive individuals for primary aldosteronism (PA).11 A further LC-MS/MS method for quantifying iothalamate in plasma and urine can accurately assess glomerular filtration rate (GFR) in early stages of renal dysfunction to screen potential kidney donors.12
Analyte multiplexing and automation deliver superior and highly reproducible data to simplify increasingly sophisticated LDT methods and support clinical test validation. HRMS using triple quadrupole MS/MS is now used to screen and quantify toxic drugs, offering novel metabolomic methods to distinguish between and eliminate candidate isomers.13 Electrospray MS/MS and a computer-assisted metabolic profiling algorithm can automatically flag abnormal profiles in newborns from a single spot of blood.14 Automated MALDI-TOF MS enables clinical microbiology laboratories to identify and classify bacteria and other microorganisms.15,16
The impact of mass spec in clinical labs
Exponential growth in MS methods in clinical laboratories is expected in high-throughput and quantitative clinical and translational workflows, bacterial identification, imaging of tissue sections, diagnostic testing, and functional assays. Improvements in automation will support validation and seamless communication with laboratory information management systems (LIMS) to bring mass spectrometry-based detection into larger and more complex clinical laboratories. The development of handheld mass spectrometers will enable clinical measurements in remote environments opening up the possibility of point-of-care applications.1
In conclusion, as technology advances and technological challenges such as sample preparation, online extraction, throughput, automation, and system interfacing are overcome, we can expect the impact of MS in clinical laboratories to mature. “Mass spec” will increasingly be relied upon for sensitive, highly reproducible, accurate results.
REFERENCES
- The Future of Clinical Mass Spectrometry – AACC.org. https://www.aacc.org/publications/cln/articles/2015/february/future-of-mass-spec.
- January issue of AACC’s Clinical Chemistry journal sheds light on how mass spectrometry is transforming clinical testing and leading to global improvements in patient health – AACC.org. https://www.aacc.org/media/press-release-archive/2016/january/clinical-chemistry-journal-sheds-light-on-how-mass-spectrometry-is-transforming-clinical-testing.
- Applications of Mass Spectrometry to the Clinical Laboratory. http://www.medscape.com/viewarticle/753445_2.
- Choices A, Vendor I. AACC / MSSS outlook for clinical mass spec testing q1 my lab is … aacc / msss outlook for clinical mass spec testing q2 currently, do you view clinical mass spec applications being used as … ( select all that apply ). 2015:1-17.
- Norris JL, Caprioli RM. Imaging mass spectrometry: a new tool for pathology in a molecular age. Proteomics Clin Appl. 2013;7(11-12):733-738. doi:10.1002/prca.201300055.
- Cutting-edge lab technology used to create labs-on-a-plate, intelligent surgical knives – AACC.org. https://www.aacc.org/media/press-release-archive/2016/january/cutting-edge-lab-technology-used-to-create-labs-on-a-plate-intelligent-surgical-knives.
- Mass spectrometry imaging used for tissue analysis | the digital pathology blog. http://tissuepathology.com/2014/01/16/mass-spectrometry-imaging-used-for-tissue-analysis/#axzz40Q2HOys2.
- Soldin SJ, Soukhova N, Janicic N, Jonklaas J, Soldin OP. The measurement of free thyroxine by isotope dilution tandem mass spectrometry. Clin Chim Acta. 2005;358(1-2):113-118. doi:10.1016/j.cccn.2005.02.010.
- Taguchi A, Hanash SM. Unleashing the power of proteomics to develop blood-based cancer markers. Clin Chem. 2013;59(1):119-126. doi:10.1373/clinchem.2012.184572.
- Psychogios N, Hau DD, Peng J, et al. The human serum metabolome. PLoS One. 2011;6(2):e16957. doi:10.1371/journal.pone.0016957.
- Carter S, Owen LJ, Kerstens MN, Dullaart RPF, Keevil BG. A liquid chromatography tandem mass spectrometry assay for plasma renin activity using online solid-phase extraction. Ann Clin Biochem. 2012;49(Pt 6):570-579. doi:10.1258/acb.2012.011186.
- Rhea JM, Ritchie JC, Molinaro RJ. Development of a liquid chromatography tandem mass spectrometry method for iothalamate measurement to assess renal function for potential kidney donation. Clin Chim Acta. 2013;420:104-108. doi:10.1016/j.cca.2012.12.003.
- Liotta E, Gottardo R, Bertaso A, Polettini A. Screening for pharmaco-toxicologically relevant compounds in biosamples using high-resolution mass spectrometry: a “metabolomic” approach to the discrimination between isomers. J Mass Spectrom. 2010;45(3):261-271. doi:10.1002/jms.1710.
- Rashed MS, Bucknall MP, Little D, et al. Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin Chem. 1997;43(7):1129-1141. http://www.ncbi.nlm.nih.gov/pubmed/9216448.
- Sauer S, Kliem M. Mass spectrometry tools for the classification and identification of bacteria. Nat Rev Microbiol. 2010;8(1):74-82. doi:10.1038/nrmicro2243.
- Meng Q. Mass spectrometry applications in clinical diagnostics. J Clin Exp Pathol S. 2013:4172. doi:10.4172/2161-0681.S6-e001.