Predictors of severe sepsis among patients hospitalized for community-acquired pneumonia
To earn CEUs, visit www.mlo-online.com under the CE Tests tab.
LEARNING OBJECTIVES
1. Discuss the health indications that led to the development of this particular study.
2. Identify inclusion and exclusion criteria in regard to the design of the study.
3. Discuss results, findings, and limitations of the study.
4. Discuss the concluding recommendations regarding patients with severe-sepsis CAP.
With an incidence of three to five cases per 1,000 adults per year, Community-acquired pneumonia (CAP), is a frequent cause of death worldwide.1-3 Severe sepsis, the syndrome of infection complicated by systemic inflammation and organ dysfunction, is a complication of CAP. Severe sepsis is a worldwide health problem, and a significant one in the United States, with an incidence of 343 cases per 100,000 inhabitants in the U.S. At least one-third of CAP patients present to the hospital with severe sepsis.4-5
Initial identification of the severity of sepsis is important in order to institute different management and monitoring measures.6 Clinicians often do not recognize the presence of severe sepsis in CAP patients, even when organ dysfunction is present. Studies aimed at identifying the CAP population at risk of developing severe sepsis in the community before arriving at the hospital are lacking.
The aim of our study was to determine the risk factors for presentation at the hospital with severe sepsis in patients with CAP.
Patients and data collection
A prospective, multi-center, observational cohort study was carried out in 13 hospitals belonging to the Spanish National Health System (CAP Quality Group); a complete, detailed description has been reported in a prior publication.7 Briefly, the inclusion criterion was a diagnosis of CAP, defined as acute symptoms or signs with a new compatible radiographic lung infiltrate. Exclusion criteria were nursing-home patients, transplant or oncologic patients, leukopenia or neutropenia (unless attributable to pneumonia), Human immunodeficiency virus-positive (HIV) patients with severe immunosuppression (CD4 <100), treatment with corticosteroids (>20 mg/day) or other immunosuppressive drugs, and patients with DNR (do not resuscitate) orders or in whom CAP was considered a terminal event. The study was approved by the ethics committee (ISS Hospital La Fe 2004/15 July, Assent 2004/0101), and the patients provided written informed consent.
We recorded data on age, gender, prior antibiotic treatment for the current episode, comorbid conditions (chronic obstructive pulmonary disease [COPD], heart, liver, neurological or renal diseases, and diabetes mellitus), clinical, analytical and radiological results, and the prognostic scales Pneumonia Severity Index (PSI)8 and CURB65 risk class.9
Comorbidities were assessed based on clinical history along with prior discharge diagnoses and clinical records, review of medications, and results of analyses.8 Sepsis and severe sepsis were evaluated at CAP diagnosis on hospital admission, following previously accepted criteria.4,7,10 Sepsis was defined as the presence of pneumonia and systemic inflammatory response syndrome (SIRS). Severe sepsis was considered if criteria for sepsis were met and acute failure of at least one organ was present: arterial hypoxemia (PaO2/FiO2 <300), creatinine >2 mg/dL, acute confusion, or hypotension (systolic arterial tension [ST] <90 mmHg). While organ dysfunction has also been defined in terms of hepatic or hematologic failure, information on these organ systems was not available in the data set.
Microbiological analysis and diagnostic criteria
Microbiological studies comprised the following: 2,550 (62.7%) blood cultures; 3,636 (89.3%) urinary antigens for Legionella pneumophila and 3,654 (89.8%) for Streptococcus pneumonia; 1,760 (43.2%) sputum cultures, 1,902 (46.7%) paired serological studies for Chlamydophila pneumoniae, Mycoplasma pneumoniae, Coxiella burnetii, and Legionella pneumophila; nasopharyngeal swabs to detect viral nucleic acids; and invasive samples obtained by bronchoscopy (285 [7%] bronchial aspirate [BAS] and 118 [2.9%] bronchoalveolar lavage [BAL]), and 276 (6.8%) pleural fluid cultures.
Microbiologic diagnostic criteria were the following: 1) positive urinary antigens for S pneumoniae and Legionella pneumophila; 2) isolation of microorganisms in BAL (≥104 UFC/mL), BAS (≥105 UFC/mL) or in pleural fluid; 3) isolation of one predominant microorganism in sputum or L pneumophila in buffered charcoal yeast extract (BCYE) agar; 4) microorganisms in blood culture; 5) seroconversion or a fourfold antibody increase in titers of IgG for C pneumoniae (≥ 1:512), M pneumoniae and C burnetii, (≥ 1:160) or IgM ≥ 1:32 for C pneumoniae, and ≥ 1:80 for M pneumoniae and C burnetii; 6) positive detection of viral nucleic acids in nasopharyngeal swab.
Mixed etiology was defined as pneumonia due to more than one pathogen (virus or bacteria).11
The evaluated outcome was mortality during hospitalization and at 30-day and 90-day follow-up. Length of stay (LOS) was defined as the number of days from hospital admission to discharge.
Data analysis was performed using the SPSS statistical software package, version 15.0. Categorical variable results were expressed as count (percentage) and were compared using the χ2 test. Continuous variables were expressed as median with interquartile range (IQR) and were analyzed using non-parametric tests. PSI and CURB65 scales were categorized as low risk (PSI ≤III/ CURB65 ≤2) and high risk (PSI >III/ CURB65 ≥3). Severe sepsis was dichotomized as yes (severe-sepsis criteria at hospital admission) and no (non-severe-sepsis criteria, the reference group).
Two multivariable statistical studies to predict risk factors for severe-sepsis CAP, the dependent variable, were performed using stepwise logistic regression analyses. In the first model, the included independent variables were those related to characteristics of patients. In the second model, the independent variables were those related to etiology (causal microorganisms). In both models, the independent variables were those found to be significant in the univariate analyses. The Hosmer and Lemeshow goodness-of-fit test was performed to evaluate the adequacy of the models.12
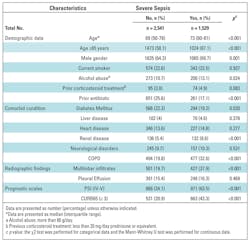
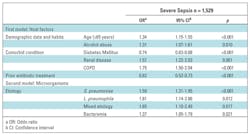
The cohort comprised 4,374 patients presenting to the emergency department with CAP and admitted to the hospital. We studied 4,070 patients after excluding 237 nursing-home and 66 DNR patients: 1,529 (37.6%) had severe sepsis (Table 1).
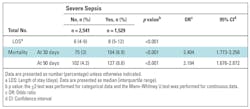
Mortality for the whole cohort was 3.3% and the median length of stay was 7 (IQR 4–10) days. Mortality was significantly higher in severe-sepsis CAP (Table 2).
Characteristics related to severe-sepsis CAP compared to the reference group are shown in Table 1. Severe-sepsis CAP was more frequent in men, patients older than 65 years, and those with COPD and renal disease, whereas diabetes mellitus was more frequent in those without sepsis. Severe-sepsis CAP also presented with higher PSI and CURB65 scores and more multilobar infiltrates. Patients who received prior antibiotic treatment had lower rates of severe sepsis.
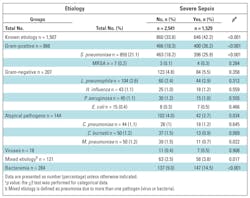
Etiological diagnosis in the whole cohort was reached in 1,506 (37%) patients: 859 (57%) S pneumoniae, 104 (6.9%) L pneumophila, 44 (2.9%) C pneumoniae, 50 (3.3%) C burnetii, 50 (3.3%) M pneumoniae, 45 (3%) Pseudomonas aeruginosa, 43 (2.9%) Haemophilus influenzae, 18 (1.2%) viruses, 15 (1%) E. coli and 121 (8%) mixed etiology.
Severe-sepsis CAP patients had the highest percentage of identified causal microorganisms and more bacteremic episodes. S pneumoniae was the most frequent microorganism found, with a higher percentage in severe sepsis. Atypical microorganisms were more frequent in patients with non-severe sepsis, whereas mixed etiology appeared more often in severe-sepsis CAP. Mixed etiology was caused mainly by S pneumoniae (29.3% with virus or atypical pathogens, 13.8% with Pseudomonas aeruginosa and 5.1% with S aureus) (Table 3).
Four independent risk factors related to patients’ characteristics were associated with severe-sepsis CAP: age >65 years, alcohol abuse, renal disease, and COPD, whereas prior antibiotic treatment and diabetes were protective factors. With regard to causal microorganisms, S pneumoniae, mixed etiology, and bacteremia were found to be risk factors (Table 4).
Discussion
The most important findings of our study were: 1) 37.6% of hospitalized CAP patients had developed community-onset severe sepsis already at admission; 2) elderly patients, alcohol abusers, patients with renal disease, and COPD patients were more likely to develop community-onset severe sepsis, whereas prior antibiotic treatment was a protective factor; 3) S pneumoniae and mixed etiology are the main causal microorganisms of severe sepsis.
Severe-sepsis CAP is not well characterized in terms of the most susceptible population even though it can appear in over one-third of the patients. We have identified the aforementioned characteristics, two of them related to comorbid conditions. However, diabetes was more frequent in those without severe sepsis, probably reflecting more lenient hospitalization criteria in diabetic patients.
At hospital admission, patients with severe-sepsis CAP had higher PSI and CURB65 scores, although more than half of these patients had a CURB65 score ≤2, pointing out the limitations of scales for severity assessment. Patients who had initiated outpatient antibiotic treatment presented a lower frequency of severe-sepsis CAP at hospital arrival. Prior studies have reported the protective effect on mortality when antibiotic therapy was rapidly initiated between four and six hours after arrival at the hospital.7,13 Prompt antibiotic administration may rapidly reduce the bacterial load, down-regulating the initial inflammatory cascade and thus decreasing the risk of sepsis.14,15 On initial severity assessment of CAP, severe-sepsis criteria should be taken into account for the decision-making process, including allocation, monitoring, and management.6
The multivariable statistical analyses results confirm that alcohol abuse and two comorbid conditions (COPD and renal disease) were independent host risk factors for developing severe-sepsis CAP in the community. The impact of alcohol on developing severe CAP has been linked to an abnormal immune response.15-18 Curiously, despite the increased risk for severe CAP in COPD patients, mortality is not higher, probably due to the use of previous antibiotics and corticosteroids that reduce inflammatory response.19-20 Our results suggest that patients with alcohol abuse, COPD, and renal diseases should be specifically targeted for preventive strategies when in contact with health systems, that is, at discharge or during scheduled outpatient visits. Moreover, if treated as outpatients for CAP, they should be closely monitored and receive instructions to rapidly recognize the signs of sepsis.
Bacteremia and etiological microorganisms are more frequently identified when CAP presents with severe sepsis, most likely due to a higher burden of pathogens in most severe episodes.2,21 S pneumoniae was the most frequently isolated microorganism in severe CAP 1,22,23 and, specifically, some serotypes have been independently associated with septic shock.24 Mixed etiology was the second-most common etiology in severe-
sepsis CAP, underscoring the impact of associated microorganisms on severity. Patients presenting with severe sepsis should benefit from optimizing microbiological tests to rule out bacteremia and mixed etiology, immediately before initiating a combination antibiotic therapy.
This study has some limitations. We have excluded the nursing-home population and patients with CAP considered a terminal event in order to avoid a different population with different characteristics, more frequent nosocomial infections, and/or multidrug resistant microorganisms and limited therapeutic efforts; therefore, our findings are not applicable to that subset of population. Second, microbiological diagnosis with regard to viruses was incomplete in a considerable subset of patients, the percentage of blood cultures was suboptimal (62.7%), and determination of S pneumoniae serotypes was not performed. The indications of microbiological tests in our study relied on the attending physicians. Third, the information regarding septic shock was not recorded. Nevertheless, our strengths are the large sample size and the prospective study design.
Conclusions
Elderly patients, alcohol abuse, and some comorbidities such as COPD and renal disease are predisposing conditions for progressing to severe-sepsis CAP in the community, mainly due to S pneumoniae and mixed etiologies. Those findings may have clinical implications for patients and physicians in primary care and emergency rooms. Preventive CAP strategies such as vaccination—influenza and S pneumoniae—and health measures recommended in guidelines should be reinforced in the most susceptible patients. Recognition of severe-sepsis CAP signals should be encouraged for patients and for physicians in primary care and/or emergency rooms. Initial severity CAP assessment could be improved by evaluation of severe-sepsis criteria at diagnosis in order to optimize microbiological and analytical tests, to provide closer monitoring and a rapid antibiotic treatment. Efforts should be directed to encouraging actions to reduce the burden of severe-sepsis CAP episodes and facilitate its prompt recognition.
References
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–9. doi: 10.1136/thx.2009.129502. pmid:20729232
- Rello J. Demographics, guidelines, and clinical experience in severe community-acquired pneumonia. Critical Care. 2008;12 Suppl 6:S2. doi: 10.1186/cc7025. pmid:19105795
- Welte T, Köhnlein T. Global and local epidemiology of community-acquired pneumonia: the experience of the CAPNETZ Network. Semin Respir Crit Care Med. 2009;30: 127–135. doi: 10.1055/s-0029-1202941. pmid:19296412
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003; 29:530–538. pmid:12664219 doi: 10.1007/s00134-003-1662-x
- Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12: R158. doi: 10.1186/cc7157. pmid:19091069
- Ewig S, Ruiz M, Mensa J, et al. Severe Community-acquired Pneumonia. Assessment of Severity Criteria. AMJ Respir Crit Care Med. 1998;158:1102–1108. pmid:9769267 doi: 10.1164/ajrccm.158.4.9803114
- Menéndez R, Torres A, Reyes S, et al. Initial management of pneumonia and sepsis: factors associated with improved outcome. Eur Respir J. 2012;39: 156–162. doi: 10.1183/09031936.00188710. pmid:21828033
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. pmid:8995086 doi: 10.1056/nejm199701233360402
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. pmid:12728155 doi: 10.1136/thorax.58.5.377
- Dremsizov T, Clermont G, Kellum JA, Kalassian KG, Fine MJ, Angus DC. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129: 968–978. pmid:16608946 doi: 10.1378/chest.129.4.968
- Cillóniz C, Ewig S, Ferrer M, et al. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit Care. 2011;15: R209. doi: 10.1186/cc10444. pmid:21914220
- Hosmer D, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons, 1989. Inc ed.
- Restrepo MI, Mortensen EM, Velez JA, Frei C, Anzueto A. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest. 2008;133: 610–617. pmid:17989157 doi: 10.1378/chest.07-1456
- Schaaf BM, Boehmke F, Esnaashari H, et al. Pneumococcal septic shock is associated with the interleukin-10-1082 gene promoter polymorphism. Am J Respir Crit Care Med. 2003;168: 476–480. pmid:12746253 doi: 10.1164/rccm.200210-1164oc
- Ruiz M, Ewig S, Torres A, et al. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160: 923–929. pmid:10471620 doi: 10.1164/ajrccm.160.3.9901107
- Mehta AJ, Guidot DM. Alcohol abuse, the alveolar macrophage and pneumonia. Am J Med Sci. 2012;343: 244–247. doi: 10.1097/MAJ.0b013e31823ede77. pmid:22173040
- Bertran MJ, Trilla A, Codina C, Carné X, Ribas J, Asenjo MA. Analysis of the cost-effectiveness relationship in the empirical treatment in patients with infections of the lower respiratory tract acquired in the community. Enferm Infecc Microbiol Clin. 2000;18:445–451. pmid:11149168
- de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest. 2010;138:994–1003. doi: 10.1378/chest.09-1425. pmid:20923804
- Liapikou A, Polverino E, Ewig S, et al. Severity and outcomes of hospitalised community-acquired pneumonia in COPD patients. Eur Respir J. 2012;39:855–861. doi: 10.1183/09031936.00067111. pmid:21920895
- Almirall J, Bolíbar I, Serra-Prat M, et al. Relationship between the use of inhaled steroids for chronic respiratory diseases and early outcomes in community-acquired pneumonia. PLoS One. 2013; 8: e73271. doi: 10.1371/journal.pone.0073271. pmid:24039899
- Menéndez R, Sahuquillo-Arce JM, Reyes S, et al. Cytokine activation patterns and biomarkers are influenced by microorganisms in community-acquired pneumonia. Chest. doi: 10.1378/chest.11-1446.
- Schaaf B, Kruse J, Rupp J, et al. Sepsis severity predicts outcome in community-acquired pneumococcal pneumonia. Eur Respir J. 2007;30:517–524. pmid:17537775 doi: 10.1183/09031936.00021007
- de Roux A, Ewig S, García E, et al. Mixed community-acquired pneumonia in hospitalised patients. Eur Respir J. 2006;27:795–800. pmid:16585087 doi: 10.1183/09031936.06.00058605
- Garcia-Vidal C, Ardanuy C, Tubau F, et al. Pneumococcal pneumonia presenting with septic shock: host- and pathogen-related factors and outcomes. Thorax. 2010;65:77–81. doi: 10.1136/thx.2009.123612. pmid:19996337
Beatriz Montull, MP, Rosario Menéndez, MD, PhD, and Raúl Méndez, MP, are members of the Pneumology Department, ISS/Hospital Universitario y Politecnico La Fe, CIBER Enfermedades Respiratorias (CIBERES), Valencia, Spain. Antoni Torrez, MD, PhD, is a member of the Pneumology Department, Hospital Clínico y Provincial, IDIBAPS, CIBER EnfermedadesRespiratorias (CIBERES), Barcelona, Spain.
Complete information about all contributing authors can be found at http://www.plosone.org/article/authors/info%3Adoi%2F10.1371%2Fjournal.pone.0145929
Citation: Montull B, Menéndez R, Torres A, Reyes S, Méndez R, Zalacaín R, et al. (2016) Predictors of Severe Sepsis among Patients Hospitalized for Community-Acquired Pneumonia. PLoS ONE 11(1): e0145929. doi:10.1371/journal.pone.0145929. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0145929.
The manuscript was lightly edited for style.