Unmasking anti-nuclear antibodies: The critical role of serial dilution
Systemic autoimmune rheumatic diseases (SARDs) comprise a number of autoimmune disorders, including systemic lupus erythematosus (SLE), systemic sclerosis (SSc), anti-phospholipid syndrome (APS), Sjögren’s syndrome (SS), idiopathic inflammatory myositis (IIM), vasculitis, and rheumatoid arthritis (RA). SARDs are characterized by autoantibody production against connective tissue, joints, and muscles, but can affect other organs such as the skin, lungs, heart, or kidneys.1,2 Despite their heterogeneity, early symptoms of SARDs—such as headache, fever, malaise, polyarthralgia, and fatigue—are often non-specific and overlapping, making diagnosis challenging based on clinical manifestations, alone.1 Therefore, supplemental laboratory testing can offer diagnostic guidance for select SARDs3,4 and corroborate clinical symptoms as physicians navigate patient diagnosis and subsequent prognostic decisions,4,5 since many autoantibodies bear specificity for a particular clinical phenotype.5
A key laboratory test that can contribute to diagnostic decisions,3 help predict clinical onset,2 and aid in the management of autoimmune disease6 is the detection of anti-nuclear antibodies, or ANAs. ANA detected in patient serum are a characteristic finding in many SARDs and some specific ANAs are included in the classification criteria for these rheumatic diseases.2-5,7 ANAs are autoantibodies directed against nuclear antigens, in addition to those binding the nuclear envelope, mitotic spindle apparatus, or cytoplasm. The clinical relevance of antibodies directed against structures outside of the nucleus, such as cytoplasm or mitochondria, outdate the term “ANA.” The International Consensus on ANA Patterns (ICAP) is actively incorporating more general, standardized nomenclature into the field, namely, anti-cell antibody codes, or AC-codes, to address this.8 AC-codes are meant to introduce pattern-naming conventions independent of the subjective language used by the international community. ICAP aims to standardize laboratory ANA screening, interpretation, and reporting through an ongoing global effort comprising internationally recognized experts dedicated to harmonizing the HEp-2 indirect immunofluorescence assay (IFA) test.9 According to ICAP, the gold standard for ANA testing is IFA performed on the HEp-2 cell substrate or variants of this cell line (i.e., HEp-20-1010 or HEp-200011).5,9 HEp-2 cells contain a broad spectrum of 100–150 cell antigens at different stages of the cell cycle, allowing for the sensitive detection of numerous clinically relevant autoantibodies, in addition to antibodies that may not have been isolated yet.12 It is recommended that HEp-2 IFA testing be followed by a confirmatory monospecific assay such as enzyme-linked immunosorbent assay (ELISA), fluorescent enzyme immunoassay (FEIA), immunoblot, or chemiluminescence immunoassay (ChLIA).12,13 This white paper will emphasize the importance of adhering to ICAP’s guidelines for ANA screening and highlight the potential consequences of using only a single dilution—focusing on the risk of overlooking mixed patterns amongst routine ANA samples.
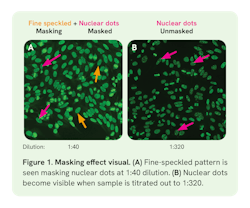
In 2021, ICAP published guidelines8 on disease-specific recommendations in addition to recommendations for reporting titers, patterns, and screening dilutions for the HEp-2 IFA.5 The guidelines state that both the antibody titer and pattern (AC-code) should be reported, since clinical relevance of the test result may be titer and pattern-dependent. It is recommended to serially dilute positive, clinically significant12 patterns to at least 1:640 or 1:1280. These additional dilutions may reveal masked ANA patterns as seen in Figure 1.8 Alternatively, computer-aided diagnostic (CAD) systems can perform end-point titer estimations using fluorescence intensity (FI) measurements at a single screening dilution, usually 1:80.14 This estimation is called a single dilution estimate. However, ICAP does not recommend reporting a titer based on FI estimates at the screening dilution if serial dilutions were not performed.5,8 While single dilution can provide information on titer estimate and likelihood of disease, its 22% error rate15 and overestimation of the titer in up to 44% of samples14 means titers may be reported incorrectly unless serial dilutions are performed.8 This limitation exists because the relationship between FI and titer is not linear5—it is system-specific14 and dependent on both substrate and ANA pattern.16,17
Although a single dilution estimate may appear to save time and reduce costs for laboratories, its actual efficiency gains are minimal. One study analyzed 400 samples/day comparing single dilution to a more comprehensive, automated three-well screening approach.18 The latter method automatically reflexed positive samples for titers at three dilutions (1:80, 1:320, 1:1,280) and found only a 16-minute difference in hands-on labor between single dilution and the three-well screen during one 8-hour shift.18 The authors state that the 16-minute time reduction with single dilution should not justify the method due to its associated risk with decreased accuracy and interferences such as the prozone or masking effect,18 which will be the focus of this white paper.
The prozone effect can occur in high-titer samples that are not sufficiently diluted, resulting in false-negatives if using only a single dilution.18 This occurs when antibody excess is present, resulting in diffuse, faint, or negative staining, which is resolved by diluting the sample. On the other hand, masking can occur when two or more antibodies are present in a sample, but evaluation of individual patterns is hindered due to the predominance of one pattern. Essentially, a masked pattern is hidden unless serial dilutions are performed to titer out the competing pattern, thereby “unmasking” these antibodies (Figure 1).18 Another consideration is that ANA specificity is low (60‒74.7%) in certain diseases like SLE at a typical screening dilution of 1:80, and likelihood of disease is known to increase with antibody titer, highlighting the importance of accurate titer reporting due to its potential prognostic value.4,7,8,12 Accordingly, ICAP recommends that reports should specify whether the titer is obtained through serial dilutions or estimated via single dilution.8
Furthermore, end-point titer estimates based on a single dilution cannot be made for mixed, cytoplasmic, or undetermined patterns, and clinically relevant mixed patterns may be masked and overlooked when utilizing such an approach.5,8,14,15,18 This presents a challenge since pattern recognition is a useful tool that may support clinical decisions, aiding in both diagnosis and prognosis.8,12,19
Simple patterns are defined as staining a single cellular domain and containing distinct attributes from other patterns (e.g., nuclear envelope, centromere).9 The term “mixed patterns”9 refers to the co-occurrence of two or more simple patterns staining the same cellular compartment, making it difficult to distinguish individual AC patterns. This should not be confused with the term “multiple patterns,”9 which refers to two or more distinguishable patterns staining distinct cellular compartments (e.g., nuclear and mitochondrial, both seen in primary biliary cholangitis5,8). Importantly, mixed patterns are particularly relevant in SARDs such as SLE, which present with several autoantibodies against antigens in the nucleus.5,9 Studies estimate the prevalence of mixed or multiple ANA patterns in SLE patients ranges from 6.5% to 15.1%.20-23 These specific patterns often only appear at the highest ANA titers, indicating a possible link to disease activity and displaying potential to serve as a prognostic marker.22,24 Hence, it is important to identify all patterns as they may each have clinical significance.12
Masked ANAs refer to antibodies that are present, but undetectable at a given dilution due to the presence of another pattern (i.e., “masking pattern”) (Figure 1). Accordingly, Carter, et al. observed the masking effect in 1% (n = 29) of routine ANA samples (n = 3,000) tested over a 6-month period in their rheumatology clinic, Lexington Medical Laboratories (personal communication). The “masking” patterns were most often high titer, homogeneous, or speckled, while the “masked” patterns exhibited more variation. This study emphasizes the importance of screening practices consistent with the ICAP-recommended guidelines9 stating that positive samples be titrated to at least 1:640 or 1:1,280 to reveal masked patterns that may hold clinical significance. This white paper presents the results and data from the Carter study at Lexington Medical Laboratories, which revealed masked patterns in 1% of samples collected during routine ANA testing.
Methodology
Anti-nuclear antibody testing via HEp-2 IFA
Routine patient samples (n = 3,000) were collected by physicians in the rheumatology clinic from a community hospital immunology laboratory (Lexington Medical Laboratories, Lexington Medical Center, West Columbia, South Carolina). Serum samples were incubated using an IFA HEp-20-10 cell kit according to the manufacturer’s protocol. HEp-20-10 cells are an improved variant of the HEp-2 cell line, which are modified in a way that results in a 10-fold increase of mitotic cells, aiding in pattern identification.10 Briefly, serum samples were diluted for initial screening at 1:40 and 1:160 in phosphate-buffered saline (PBS)-Tween and subject to washing and conjugate incubation steps before initial evaluation. The 1:40 dilution was used based on the manufacturer’s instructions. The additional 1:160 dilution helps to reduce potential false negatives from the prozone effect and uncover masked ANA patterns. Slides containing HEp-20-10 cells were processed and incubated using automation. Positive samples from screening dilutions were reflexed for titers at additional dilutions and titrated to endpoint. Samples were analyzed and interpreted using a fully automated microscope and associated software, in addition to interpretation by two technologists experienced in fluorescent microscopy.
Qualitative determination of antibodies using monospecific tests
ELISA profiles were utilized to confirm HEp-20-10 IFA results. The following profiles were used according to the manufacturer’s instructions: ENA Profile (IgG) against nRNP/Sm, Sm, SS-A, SS-B, Scl-70, Jo-1, CENP B, and ribosomal P-proteins, Autoimmune Liver Disease Profile (IgG) against AMA-M2, M2-3E, Sp100, PML, gp210, LKM-1, LC-1, SLA/LP, and Ro-52, Systemic Sclerosis Profile (Nucleoli) against Scl-70, CENP A, CENP B, RP11, RP155, Fibrillarin, NOR90, Th/To, PM-Scl100, PM-Scl75, Ku, PDGFR, and Ro-52. Briefly, using an automated system, strips were pretreated with sample buffer, incubated with diluted patient serum samples, washed with wash buffer, and then incubated with an enzyme-labelled anti-human IgG enzyme conjugate to catalyze a color reaction that was then measured using a flatbed scanner and evaluated with associated software as per the manufacturer’s protocol.
Results
Among 3,000 routine ANA samples tested in a 6-month period, 29 (or 1%) had distinct masked patterns. Here, we show four examples of masked ANA IFA patterns. “Masking” patterns were mainly high-titer, speckled, or homogenous, whereas “masked” patterns included nucleolar, centromere, cytoplasmic M2, lysosomal, or NOR-90 patterns. Importantly, these patterns would have been missed by a single dilution IFA screening protocol. ENA line blot profiles are used to detect specific sets of antibodies, particularly ones present in the classification criteria of multiple rheumatic conditions (i.e., anti-SS-A/Ro-52 in Sjögren’s syndrome).25
Case 1: Masked centromere pattern (AC-3)
Case 1 included an 81-year-old female patient with Raynaud’s syndrome, vasculitis, and dysphagia-CREST syndrome. ANA positivity was seen in both the 1:40 and 1:160 screening dilutions (Figure 2A‒B), showing diffuse nuclear and non-specific cytoplasmic speckled staining, prompting further dilution. The titer of the nuclear speckled pattern (AC-4/31) was 1:2,560. Further dilution revealed centromeres (AC-3) at 1:640, which by 1:2,560 was unmasked and had become the dominant pattern with a titer of 1:10,240 (Figure 2C‒D). The ENA profile was positive for anti-CENP-B antibodies against centromeres, confirming the masked AC-3 pattern.
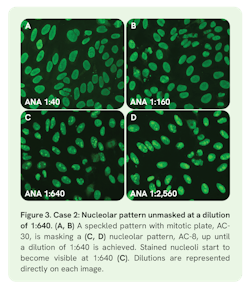
Case 2: Masked nucleolar pattern (AC-8)
Case 2 involved a 65-year-old female patient presenting with clinical features of Sjögren’s syndrome. Screening titers revealed a nuclear speckled pattern with mitotic plate positivity (AC-30) with increasing fluorescent signal from 1:40 to 1:160, prompting further dilutions (Figure 3A‒B). The titer of the AC-30 pattern was 1:1,280. While serially diluting the first pattern to endpoint, a masked nucleolar pattern (AC-8) emerged at the 1:640 dilution (Figure 3C) with a titer of 1:10,240. The ENA profile revealed autoantibodies against SS-A and Ro52, which confirmed the nuclear speckled pattern with mitotic plate staining (AC-30).
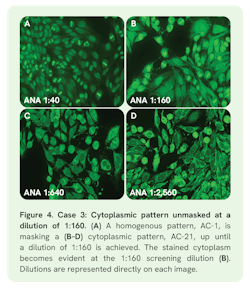
Case 3: Masked cytoplasmic speckled pattern (AC-21)
Case 3 presents a 65-year-old female with false positive syphilis (STS), abnormal liver enzymes (LFTs), and a negative hepatitis profile. ANA screening at 1:40 exclusively showed a homogenous pattern (AC-1) (Figure 4A). Yet, at the 1:160 screening dilution a masked cytoplasmic reticular/anti-mitochondrial antibody (AMA)-like pattern (AC-21) started to emerge (Figure 4B). While the titer of the AC-1 pattern was 1:160, the AC-21 pattern became dominant at dilutions greater than 1:160 and its titer was 1:5,120 (Figure 4). An autoimmune liver disease ELISA profile was positive for anti-M2 and -M2-3E, which are associated with the masked AMA-like pattern, AC-21.
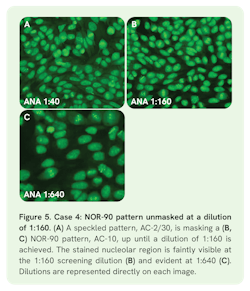
Case 4: Masked NOR-90 pattern (AC-10)
Case 4 involved a 75-year-old female with hypothyroidism and osteoarthritis, presenting with pain in the right hand and wrist. ANA screening at 1:40 resulted in a nuclear speckled pattern with mitotic plate staining (AC-2/30), titrated to an endpoint of 1:640 (Figure 5 A,C). The 1:160 screening dilution started to reveal a masked nucleolar NOR-90 (nucleolar organizer region) AC-10 pattern (Figure 5B) with a titer of 1:1,280. The systemic sclerosis ELISA profile revealed antibodies against nucleolar NOR-90, which confirmed the masked punctate nucleolar AC-10 pattern.
Conclusions and discussion
We emphasize here the importance of serial dilutions and how mixed patterns might be masked, and therefore overlooked, in ANA HEp-2 IFA screening at a single dilution. Serial dilutions can help differentiate mixed patterns in addition to unmasking hidden patterns that may provide context in the diagnosis of rheumatic disease.8,18 Approximately 1% of routine ANA samples tested in this 6-month period had distinct masking patterns that were predominantly high-titer homogenous or speckled, with masked patterns being more heterogenous. These unique and varied masked patterns would have been missed if positive samples were not titrated using serial dilutions based on the recommended guidelines published by ICAP.
In the data we present here, the identification of certain antibodies can guide clinicians towards a potential diagnosis when combined with clinical findings. For example, in Case 1, a centromere pattern (AC-3) was unmasked at a dilution of 1:640 and the corresponding anti-CENP-B autoantibodies have very high prevalence (80‒95%) for the limited form of progressive systemic sclerosis.12,26 The patient in Case 2 had clinical features of Sjögren’s syndrome, which is consistent with the observed speckled with mitotic plate AC-30 pattern and autoantibodies against SS-A and Ro52.4,12 With additional dilutions, a nucleolar pattern (AC-8) was unmasked in this sample, for which the causative antibodies were not identified by monospecific testing, underscoring the utility in performing serial dilutions on positive HEp-2 IFA samples to unmask patterns. Consistent with the literature, combining IFA with monospecific tests provides the highest clinical utility.4 Moreover, HEp-2 IFA can detect antibodies that monospecific tests may miss (as seen in Case 2), while monospecific tests can identify antibodies that IFA may miss. In patient 3, a cytoplasmic pattern was unmasked at a higher dilution, which would have been missed if only a single dilution was used. The presence of mitochondrial autoantibodies was then confirmed via a specific ELISA profile. These antibodies are highly prevalent (95%) in primary biliary cholangitis and may have been useful in supporting the patient’s clinical symptoms.5 Finally, in Case 4, unmasked antibodies against nucleolar NOR-90 (AC-10) are rare but have relevance in the diffuse form of progressive systemic sclerosis.4 In some cases, the IFA pattern results led researchers to perform unique ELISA profiles, which contained antigens that may not have been tested for otherwise, as in Cases 3 and 4.
We highlight the need to perform serial dilutions instead of estimating titers using single dilution methods, which can provide inaccurate estimates of the titer and risks overlooking masked patterns.8 For context, in the United States, the prevalence of ANAs has increased considerably in recent years—in 2012 this number was 15.9%, corresponding to approximately 41 million affected individuals.27 If we extrapolate the Carters’ (and previous14) findings to this dataset, this suggests up to 1%—or nearly 410,000 patients across the United States—might experience a delay in diagnosis or worse prognosis due to overlooking the simple titration of positive samples. Certainly, large reference laboratories, clinics, and hospitals are screening many more samples at present, over 10 years later. This volume highlights the need for a harmonized effort in ANA screening. Taken together, laboratories that adopt the strategy of titrating positive samples to at least 1:640,9 as opposed to using the single dilution method, may provide more comprehensive and accurate ANA testing, ultimately improving patient care and outcomes.
REFERENCES
- Goldblatt F, O’Neill SG. Clinical aspects of autoimmune rheumatic diseases. Lancet. 2013;382(9894):797-808. doi:10.1016/S0140-6736(13)61499-3.
- Bizzaro N. Autoantibodies as predictors of disease: the clinical and experimental evidence. Autoimmun Rev. 2007;6(6):325-333. doi:10.1016/j.autrev.2007.01.006.
- Andrade LEC, Damoiseaux J, Vergani D, Fritzler MJ. Antinuclear antibodies (ANA) as a criterion for classification and diagnosis of systemic autoimmune diseases. J Transl Autoimmun. 2022;5(100145):100145. doi:10.1016/j.jtauto.2022.100145.
- Bossuyt X, De Langhe E, Borghi MO, Meroni PL. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat Rev Rheumatol. 2020;16(12):715-726. doi:10.1038/s41584-020-00522-w.
- Bonroy C, Vercammen M, Fierz W, et al. Detection of antinuclear antibodies: recommendations from EFLM, EASI and ICAP. Clin Chem Lab Med. 2023;61(7):1167-1198. doi:10.1515/cclm-2023-0209.
- Prado MS, Dellavance A, Rodrigues SH, Marvulle V, Andrade LEC. Changes in the result of antinuclear antibody immunofluorescence assay on HEp-2 cells reflect disease activity status in systemic lupus erythematosus. Clin Chem Lab Med. 2020;58(8):1271-1281. doi:10.1515/cclm-2019-0638.
- Damoiseaux J, van Beers J. Autoantibodies to dsDNA in the diagnosis, classification and follow-up of patients with systemic lupus erythematosus. J Transl Autoimmun. 2023;6(100191):100191. doi:10.1016/j.jtauto.2023.100191.
- von Mühlen CA, Garcia-De La Torre I, Infantino M, et al. How to report the antinuclear antibodies (anti-cell antibodies) test on HEp-2 cells: guidelines from the ICAP initiative. Immunol Res. 2021;69(6):594-608. doi:10.1007/s12026-021-09233-0.
- Andrade LEC, Klotz W, Herold M, et al. Reflecting on a decade of the international consensus on ANA patterns (ICAP): Accomplishments and challenges from the perspective of the 7th ICAP workshop. Autoimmun Rev. 2024;23(9):103608. doi:10.1016/j.autrev.2024.103608.
- Rohwäder E, Locke M, Fraune J, Fechner K. Diagnostic profile on the IFA 40: HEp-20-10 - an immunofluorescence test for reliable antinuclear antibody screening. Expert Rev Mol Diagn. 2015;15(4):451-462. doi:10.1586/14737159.2015.993612.
- Keech CL, McCluskey J, Gordon TP. Transfection and overexpression of the human 60-kDa Ro/SS-A autoantigen in HEp-2 cells. Clin Immunol Immunopathol. 1994;73(1):146-151. doi:10.1006/clin.1994.1181.
- Damoiseaux J, Andrade LEC, Carballo OG, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis. 2019;78(7):879-889. doi:10.1136/annrheumdis-2018-214436.
- Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73(1):17-23. doi:10.1136/annrheumdis-2013-203863.
- Van Hoovels L, Schouwers S, Van den Bremt S, et al. Analytical performance of the single well titer function of NOVA View®: good enough to omit ANA IIF titer analysis? Clin Chem Lab Med. 2018;56(11):258-261. doi:10.1515/cclm-2018-0338.
- Won DIL. Measurements of endpoint titers based on the fluorescence intensity trend in anti-nuclear antibody testing. Lab Med. 2020;51(5):469-477. doi:10.1093/labmed/lmz087.
- Dellavance A, Cruvinel W de M, Francescantonio PLC, et al. Variability in the recognition of distinctive immunofluorescence patterns in different brands of HEp-2 cell slides. J Bras Patol Med Lab. 2013;49(3):182-190. doi:10.1590/s1676-24442013000300005.
- Bossuyt X, Cooreman S, De Baere H, et al. Detection of antinuclear antibodies by automated indirect immunofluorescence analysis. Clin Chim Acta. 2013;415:101-106. doi:10.1016/j.cca.2012.09.021.
- Ricchiuti V, Adams J, Hardy DJ, Katayev A, Fleming JK. Automated processing and evaluation of anti-nuclear antibody indirect immunofluorescence testing. Front Immunol. 2018;9:927. doi:10.3389/fimmu.2018.00927.
- Campochiaro C, Derrett-Smith EC. Autoantibodies in autoimmune rheumatic disease. Medicine (Abingdon). 2018;46(2):78-83. doi:10.1016/j.mpmed.2017.11.003.
- Al-Mughales JA. Anti-nuclear antibodies patterns in patients with systemic lupus erythematosus and their correlation with other diagnostic immunological parameters. Front Immunol. 2022;13:850759. doi:10.3389/fimmu.2022.850759.
- Frodlund M, Dahlström O, Kastbom A, Skogh T, Sjöwall C. Associations between antinuclear antibody staining patterns and clinical features of systemic lupus erythematosus: analysis of a regional Swedish register. BMJ Open. 2013;3(10):e003608. doi:10.1136/bmjopen-2013-003608.
- Ramachandran A, Kumar K, Sankaralingam R, Chinnadurai S, Chilukuri B. A retrospective study on antinuclear antibody patterns in systemic lupus erythematosus patients and its correlation with serological markers. Cureus. 2023;15(12):e50049. doi:10.7759/cureus.50049.
- Choi MY, Clarke AE, St Pierre Y, et al. Antinuclear antibody-negative systemic lupus erythematosus in an international inception cohort. Arthritis Care Res (Hoboken). 2019;71(7):893-902. doi:10.1002/acr.23712.
- Prado MS, Dellavance A, Rodrigues SH, Marvulle V, Andrade LEC. Changes in the result of antinuclear antibody immunofluorescence assay on HEp-2 cells reflect disease activity status in systemic lupus erythematosus. Clin Chem Lab Med. 2020;58(8):1271-1281. doi:10.1515/cclm-2019-0638.
- Yeo AL, Ojaimi S, Le S, Leech M, Morand E. Frequency and clinical utility of antibodies to extractable nuclear antigen in the setting of a negative antinuclear antibody test. Arthritis Care Res (Hoboken). 2023;75(7):1595-1601. doi:10.1002/acr.24990.
- Hudson M, Mahler M, Pope J, et al. Clinical correlates of CENP-A and CENP-B antibodies in a large cohort of patients with systemic sclerosis. J Rheumatol. 2012;39(4):787-794. doi:10.3899/rheum.111133.
- Dinse GE, Parks CG, Weinberg CR, et al. Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol. 2022;74(12):2032-2041. doi:10.1002/art.42330.
About the Author

Danielle Cervasio, PhD
is an Associate Scientific Affairs Liaison at Euroimmun US, where she curates scientific relationships and develops scientific content for endocrinology test systems, chemiluminescent-based assays, and more. She obtained her doctorate in Molecular and Cellular Pharmacology from Stony Brook University in 2023.