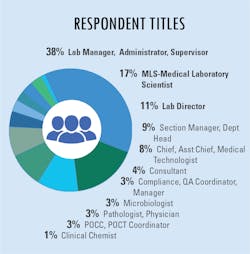
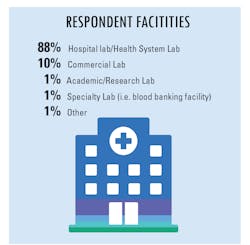
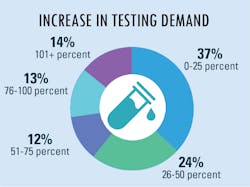
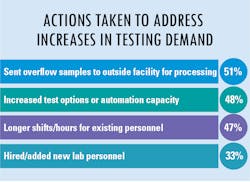
As more suspected cases are reported, increased testing demands seek to confirm infections and guide the best practices to treat patients and their symptoms. Because of this year’s global severity of the SARS-CoV-2 virus and its resulting COVID-19 disease, clinical labs were presented with a new challenge – performing tests that began with numbers in the hundreds which have now reached into the millions.
In our fourth and final “State of the Industry” survey topic for 2020, Medical Laboratory Observer (MLO) focused how labs are dealing with increased testing demands, the types of tests chosen and/or preferred by clinical labs, and how the emergence of the SARS-CoV-2 virus and the subsequent COVID-19 disease impacts daily lab procedures.
Increased lab testing demands
As lab testing demands have increased and continue to grow at a staggering rate, we asked how labs are addressing these new demands. The most common answer was to send the overflow samples to an outside facility for processing – the choice of approximately half of survey respondents. Following as a close second choice was to increase test options or automation capacity for higher throughput. Additional replies indicated that some labs chose to address the problem in-house as much as possible with longer shifts/hours for existing personnel and/or hire/add new lab personnel.
Because the testing demands have grown at exponential rates for some labs, some concerns have also emerged about the level of confidence laboratorians have in the accuracy of COVID-19 tests.
In agreement with Scinto is Linda Minnich, SM(NRM-ASCP), MS, Operations Manager Virology at Charleston Area Medical Center (CAMC), located in Charleston, WV. She summed up the issue of test confidence by saying, “Some are good and perform as claimed. Others have had issues that became obvious as they were utilized more. The limited testing for the FDA EUA has required more evaluation by the users at a time when they needed to get moving.”
Lab supplies and shortages
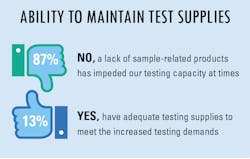
The overwhelming majority of survey respondents indicated they did have, and possible still do have, difficulty in maintaining a supply of testing products to meet their increased lab testing demands. On the other hand, a small percentage of replies indicated they have adequate testing supplies to meet the daily demands placed upon their lab personnel.
However, not all items currently in need are items that can be easily ordered from a manufacturer or supplier. Lately, labs have found that the things that would matter the most during the current pandemic are things that are the hardest to come by.
Minnich added, “CAMC has a great virology staff who have really stepped up to the plate for patient care. We have an M2000 and Panther, but reagents and supplies have been the issue. This week, no tips or deep wells, next week it is reagents. It has been very challenging to keep up with what we have, what is ordered and what is not coming.”
Test preferences and influences
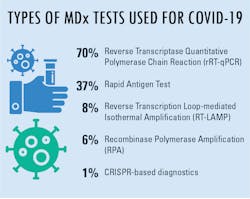
When asked about lab preference for molecular diagnostics tests being used in COVID-19 lab testing, reverse transcriptase quantitative polymerase chain reaction (rRT-qPCR) was clearly the most popular reply, followed by rapid antigen tests. In further inquiries, MLO wanted to know the main reason these were the top two answers for the majority of responses.
Additional factors that could play an important role in the choice of lab tests and equipment in the future, according to Nikos Pavlidis, VP/GM of Molecular Diagnostics for BD, located in Sparks, MD, include a nod to automation among other considerations.
“The key points here are that your categories of accuracy, speed to result, and costs all are relevant factors, but we would add automation/ease of use/workflow simplicity and open vs. closed systems, which may be additional categories to consider for the future.”
When considering reasons to use one test over another, J. Kent Morgan, PhD, Chief Science Officer at Veo Diagnostics, located in Loveland, OH, asserted, “It is important to consider the value of information that results from each test type. For instance, molecular tests can only provide an answer to the question of whether or not an individual was at the moment of sampling infected with the SARS-CoV-2 virus. This effectively limits the value of molecular testing to ‘currently sick’ people to either validate or rule out an individual as having COVID-19.”
Morgan continued, “Conversely, antibody tests can be used to help show whether a person or group of individuals has a current or past immune response to the SARS-CoV-2 virus. Antibody tests can be used to screen for immune response and possible disease prevalence in both individuals and larger populations. Antibody test results could be valuable to help understand and make informed decisions related to disease management for an individual or a larger community of people.”
Questionable results and procedures
When asked what steps – if any – labs are taking to reduce the number of potential false positive test results, nearly one-third of replies suggested that lab personnel repeat the test with another method and compare the results, while an equal third asserted that laboratorians verify all pre-analysis steps to ensure they are performed correctly.
Perhaps the most surprising of all is that among survey respondents, a fair amount admitted they are doing nothing to prevent or reduce potential false positive test results, which calls into question the issue of test result accuracy and reliability.
Scinto points out, “Previously, we relied on FDA 510(k) approved assays, which underwent several years of manufacturer performance validation. Now, laboratories are making testing decisions with SARS-CoV-2 assays that underwent several weeks of validation with FDA EUA (emergency use authorization) approval. A fair number of assays have since undergone FDA clarification and revisions due to analytical and performance issues that arose in the field. A diminished level confidence in the assays and the manufacturer was the outcome. To circumvent this, many laboratories opted to create LDT assays and apply for EUA certification. Thus, allowing them to internally validate and understand firsthand the performance characteristics of the assay and the test results they are reporting.”
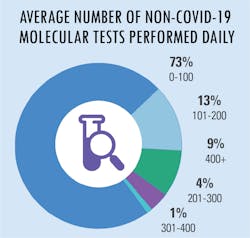
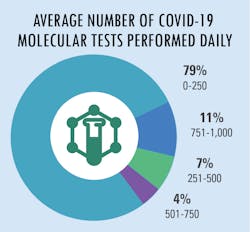
All in a day’s testing
New regulations for test reporting
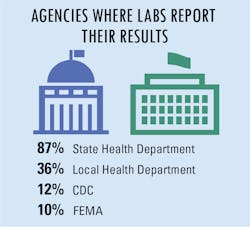
In addition, a small group of additional agencies have also been assigned to collect COVID-19 test data as well, including central labs, assigned county agencies, the U.S. Department of Health and Human Services (HHS), The Joint Commission, assigned universities, and the U.S. Department of Veteran’s Affairs.
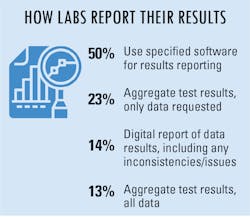
To make reporting test results easier, many labs/lab managers are taking advantage of reporting options made available to them. Half of survey respondents indicated using specified software for results reporting, while others aggregate test results and send the data collected or requested of them.
Looking to the future
According to Michelle Bosch, Senior Director, Global Commercial Marketing, Diagnostics, at Meridian Bioscience, located in Cincinnati, OH, “There is a critical need to deliver quality diagnostic solutions that are flexible and scalable, as healthcare systems continue preparing for increased testing capacity during this unprecedented time. Supply chain optimization must be a priority to continue delivering all essential diagnostic products to support our healthcare systems in providing the necessary care and treatment to patients.”
Adding to Bosch’s comment, Morgan from Veo Diagnostics said, “Test accuracy should be an area of focus for Molecular Diagnostics and the primary concern of any diagnostic lab environment. The result of accurate testing has shown to produce more reliable data, which can help communities make smarter decisions on structuring their environment.”
Pointing out an unknown but potentially serious impending issue, Michelle Tabb, PhD, Chief Scientific Officer at DiaSorin Molecular, located in Cypress, CA, said:
“Perhaps the most important area to keep in mind is the impact flu may have on the upcoming respiratory season while COVID-19 is still present. With similar symptoms and different treatment options, it will be critical for labs to ensure they can detect and differentiate between SARS-CoV-2, Flu A, Flu B and potentially RSV, so that patients get the correct diagnosis and treatment.”