KSL | Pulse Scientific announced national distribution of the over-the-counter combination test for COVID-19 and Flu, packaged and designed for self-testing at home.
Developed by Lucira Health Inc., the test has been approved by Health Canada and is now available to institutions, healthcare providers, agencies and consumers in time for the fall and winter COVID and Flu season.
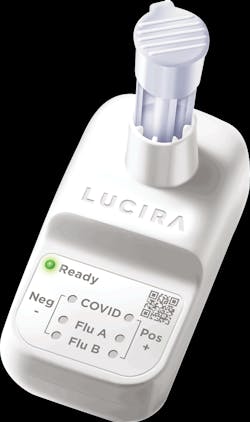
Lucira’s single-use test fits in the palm of a hand, runs on two AA batteries, and with one nasal swab, provides a positive or negative result for COVID-19, Flu A, and Flu B in 30 minutes or less. Each Lucira test is packaged with everything needed for the test. There is no separate reader or instrument to purchase and maintain.
The Lucira testing platform is a Nucleic Acid Amplification Test (NAAT) with sensitivity and specificity comparable to lab-based PCR assays. The Lucira COVID-19 & Flu Test, which uses the same technology platform, was granted FDA EUA for point-of-care use earlier this month.
Health Canada has granted authorization under Interim Order No. 3. for emergency use and commercialization of this at-home test for COVID & Flu. Health Canada’s decision is based on performance data including clinical trial results demonstrating comparable sensitivity and specificity to leading lab-based PCR assays.