The anatomy of OSHA’s Bloodborne Pathogen standard
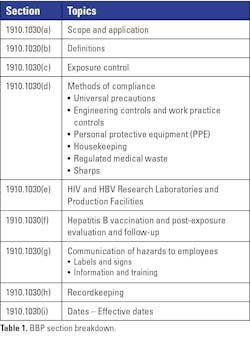
Whether you are a safety professional or drafting your first bloodborne pathogen exposure control plan, you will inevitably need to reference OSHA’s Bloodborne Pathogen (BBP) standard. The standard can be challenging to navigate, as its requirements are not always laid out in a linear fashion. Readers often need to jump between sections to fully understand their intent. This article summarizes the key components of the nine primary sections (see Table 1) and provides guidance to help ensure compliance.
Scope and application
The first section, Scope and application, simply states that OSHA’s BBP standard, codified as 29 CFR 1910.1030, is applied to all employees with an occupational exposure. The purpose is simple yet comprehensive: to identify and mitigate occupational exposure risks, thereby preventing transmission of bloodborne diseases such as HBV, HCV, and HIV. But how can an employer determine if their staff falls under this regulation? The answer lies in the standard’s next section.
Definitions
Section 2, Definitions, lays the groundwork for compliance by specifying key terms. One of which is occupational exposure: any reasonably anticipated skin, eye, mucous membrane, or parenteral contact with blood or other potentially infectious material (OPIM) that may result from the performance of an employee's duties.
What makes this definition so important is that the entire standard is based off how an employer determines if their employee has this risk in their work areas.
Exposure control
At the heart of OSHA’s standard is the Exposure Control Plan (ECP) — a living document pivotal to preventing workplace infection. In the third section, OSHA states what it takes for employees to operate in an unsafe environment safely. The BBP standard mandates that the employers’ ECP list tasks and job classifications where exposure may occur, along with what preventative steps must be in place, identified as Methods of compliance (this just so happens to be the next section of the BBP standard). The lab is a dynamic environment. Therefore, OSHA requires employers to review and update their ECP annually and when changes are made in the lab that redefine the risks in the lab.
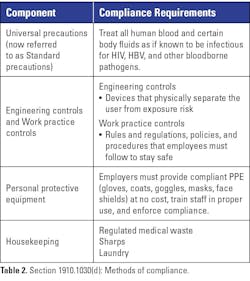
Methods of compliance
The fourth section of the standard, Methods of compliance, is one of the larger segments covering various practices employees must follow to keep themselves safe (see Table 2). So, what must an employer do to help protect staff? The best method of compliance is to utilize universal precautions (now referred to as standard precautions), “an approach to infection control... where all human blood and certain human body fluids are treated as if known to be infectious for HIV, HBV, and other bloodborne pathogens.”
Engineering and work practice controls are two crucial components of the “Methods of compliance” section. Engineering controls refer to items that physically isolate or remove the risk of working with bloodborne pathogens. They can be as complex as a biological safety cabinet or as simple as a sharps container. Work practice controls are rules and regulations and steps taken by employees that reduce the likelihood of exposure by altering the way a task is performed (e.g., prohibiting recapping of needles by a two-handed technique). OSHA also requires the employer to provide handwashing facilities or an antiseptic hand cleanser that employees must use as soon as possible after removing their PPE.
In the middle of the “Methods of compliance” section are OSHA’s requirements pertaining to PPE. Here, the standard mandates that appropriate PPE shall be offered to employees at no cost when there is a risk of occupational exposure. The standard also requires employers to ensure staff are trained how to use their PPE and enforce its use. This section elaborates on gloves, masks, eye protection, and face shields. When selecting PPE, refer to the specific requirements for each type to ensure an OSHA compliant item is selected.
The Housekeeping portion of Methods of compliance provides guidance on regulated medical waste (RMW), sharps, and contaminated laundry. OSHA considers all work surfaces that come in contact with blood or other potentially infectious materials to be contaminated. Decontamination must be completed with an approved disinfectant immediately after a spill and at the end of the shift. As with PPE, OSHA has strict requirements when selecting containers for RMW and sharps. OSHA also describes how these types of waste must be stored and moved off site in ways that reduce the risk of exposure to staff. Finally, if a facility decides to launder contaminated items such as PPE or linens, OSHA provides specific handling steps to keep these items isolated from the user and environment.
HIV and HBV research laboratories
The fifth section of the standard is specific to research laboratories that experiment with or manipulate hepatitis B and HIV. It does not apply to clinical laboratories that analyze samples that may contain HIV or Hepatitis B. These research facilities must adhere not only to the regulations mentioned in the other sections of the standard, but also to this particular section of enhanced standards around decontamination, biosecurity, and training.
Hepatitis B vaccination and post-exposure protocol
The sixth section covers requirements about hepatitis B vaccination and post-exposure evaluation and follow-up should an employee become exposed. OSHA requires employers to offer the hepatitis B vaccine and vaccine series to all staff with occupational exposure. It also mandates that there be post-exposure evaluation and follow up and prophylaxis that are available at no cost to the employee if an exposure occurs. These actions not only protect affected workers but also demonstrate an employer’s commitment to regulatory compliance and employee health.
Communication of hazards to employees
Effectively communicating hazards is essential. This is accomplished through signs, labels, and training. OSHA requires employers to clearly label all containers holding blood, other potentially infectious materials, or contaminated items (including waste containers and refrigerators) with the approved biohazard symbol in an orange or orange-red color.
OSHA also requires employers to maintain and update training programs that explain risks, safeguards, and procedures for exposure response. This training must take place at the time of hire or assignment to an area where occupational exposures exist, and then annually. Staff awareness is non-negotiable—OSHA mandates these steps to ensure every employee understands the risks and proper responses, regardless of familiarity or previous experience. To ensure this, the training must include a segment where staff have a chance to ask questions.
Recordkeeping
Thorough recordkeeping practices are the organization’s best defense during an audit or regulatory review. Employers are required to maintain training records for three years that include the date of training, the contents covered, the trainer's name and qualifications, and a list of attendees. If an employee is exposed or experiences a sharps injury, there are many other items the employee must capture, along with an extended record retention requirement of 30 years after the employee leaves the organization.
Dates
The closing section of the standard catalogues the effective date of the regulation and all subsequent amendments or revisions. Staying informed about updates is crucial, as the regulatory landscape can and does evolve in response to emerging workplace hazards or technological advances.
Conclusion
While OSHA’s Bloodborne Pathogen Standard can feel dense and non-linear, its purpose is clear: to safeguard employees from preventable occupational exposure risks. By developing and maintaining a strong exposure control plan, training employees thoroughly, enforcing proper use of PPE, and documenting compliance, employers can align with OSHA’s requirements and protect their staff.
About the Author

Jason P. Nagy, PhD, MLS (ASCP)CM,QLS
is the Laboratory Safety Support Coordinator at Sentara Health, a multi-hospital system in Virgina and North Carolina. Jason brings almost 20 years of laboratory experience to the lab as a medical laboratory scientist (MLS) and more recently in laboratory safety and education roles.