Preventing cross contamination in an infectious disease testing laboratory
In the context of an infectious disease testing laboratory, cross contamination can be defined as an accidental introduction of an extraneous material into the specimen or laboratory instruments. A few examples of such extraneous material are infectious agents such as bacteria, viruses, or a specimen of another patient.
Though limited data exists on the incidence of cross contamination in clinical laboratories, cross contamination does occur and consequently the affected laboratory witnesses an increase in false positive results. There have been a few reports of increased false positive results due to cross contamination. A report was presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) in Lisbon, Portugal during August 2022 about an incidence of cross-contamination of COVID-19 samples in a PCR lab in Belgium resulting in a high rate of false positive results. The cross-contamination occurred due to the lab personnel’s failure to change gloves.1
In a meta-analysis of data from 31 studies that evaluated 29,839 tuberculosis (TB) cultures, the researchers found that 2% (95% confidence intervals [CI] 1–2%) of all positive TB cultures are false positives caused due to laboratory cross-contamination. Moreover, 9.2% (91/990) of all patients with a preliminary diagnosis of TB had false-positive results and received unnecessary and potentially harmful treatments.2
In 2006, the Centers for Disease Control and Prevention (CDC) reported an incidence of cross-contamination of clinical specimens with Bacillus anthracis in two Idaho hospital laboratories that were conducting proficiency tests. It triggered brief concern about a potential anthrax attack.3 Thus, cross contamination can have several damaging consequences. It may not only damage the reputation of the laboratory but also badly affect the laboratory operation. Laboratory testing is suspended until the laboratory is totally decontaminated leading to delay in sending out results. Samples that were being processed are to be re-tested after the laboratory is back up and running. Hence, following good laboratory practices and taking measures to prevent cross contamination is the best path to take. Some of the measures to prevent cross-contamination episodes are as below.
Proper laboratory design
To minimize chances of errors, mix-ups, and contamination, laboratories should be designed such that the flow of the sample processing is unidirectional. The area where samples are received and accessioned should be separate from the area of reagent preparation and sample processing and analysis. Reagent preparation should be carried out in the clean room.
Clinical laboratories performing molecular testing especially the highly sensitive PCR-based assays, should take extreme care in facility design and operation to avoid sample-to-sample contamination from carry-over of nucleic acid from previous amplification of the same or similar target.
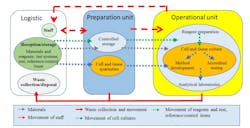
Figure 1 illustrates how the flow of materials, staff, and waste should be organized to separate processes and minimize incidence of errors and cross contamination.4 It is recommended that each area have its own dedicated storage facilities so as to avoid mixing up test items and/or reagents. And each designated area should have separate labware, lab coats, gloves, equipment (e.g., pipettes, laminar flow hood, refrigerators etc.). The clean reagent preparation room should have positive air pressure to keep contaminants out whereas the room where PCR is carried out should be under negative air pressure to keep amplicon contaminants in.Following good laboratory practice of aseptic technique by laboratory professionals
It is the responsibility of all laboratory professionals to follow good laboratory practices such as ensuring that the correct workflow is followed and they are trained to take necessary precautions to follow aseptic technique and minimize contamination and cross-contamination. When performing microbiological or molecular analysis, it is necessary to follow standard aseptic technique. This requires that all materials are sterilized and care is taken not to introduce new microorganisms from the environment into cultures.
Aseptic procedures are as follows:
- Not eating, drinking, or smoking in the laboratory.
- Wearing a disposable lab coat (that should not be taken out of the lab). If it gets contaminated with biologicals, it must be disposed of properly as a regulated medical waste.
- Washing hands before and after handling microorganisms.
- Wearing disposable gloves and safety goggles when handling cultures.
- Using disinfectants to clean the bench top before and after use.
- Washing hands with soap and water upon removing gloves and disposing of them properly.
Procedures for proper handling of bacteria stocks, cultures, and media are as follows:
- Preparing the work area by cleaning the bench top with disinfectants.
- Sterilizing the loop or needle before and after transferring bacteria.
- Only partially lifting the plate cover to prevent the introduction of foreign bacteria from the air.
- Flaming the tops of tubes before and after the transfer of microorganisms.
- In case of culture spillage, the area should be flooded with disinfectant immediately.
- Cultures should not be taken out of the laboratory.
A major source of PCR contamination in a molecular testing laboratory is aerosolized PCR products, which being small, travel and easily spread all over benches and equipment. The aerosolized PCR product then finds its way into a PCR reaction and becomes amplified. Laboratory professionals should use aerosol-free/aerosol filter pipette tips when working with PCR assays to prevent aerosol formation.
If laboratories performing PCR/real-time PCR testing discard all amplified products without opening the reaction tubes or using sealed plates can minimize the chance of contamination.5 Also, aseptic cleaning before and after PCR work of all work surfaces — bench tops, pipettors, and all touch points are necessary to prevent cross contamination.
Following good laboratory practice handling patient samples
The utmost care must be taken when handling patient samples. The person drawing the sample should wear clean gloves, a mask, and head cover. Samples should be collected using sterilized or clean sampling tools in sterile or clean tightly sealed, leak-proof containers. The sample should be labeled appropriately using two identifiers. The sample should be maintained in the recommended environment until it is analyzed.
For sample analysis, samples should be kept and accessioned in the designated dirty room. Care should be taken to prevent aerosol formation. If sample extraction needs to be carried out prior to analysis, as is the case with samples for molecular testing, it should be performed in a separate area. The addition of the sample in the reagent mix should be done aseptically in the laminar flow hood.
Care should be taken to avoid splashing of samples during liquid dispending with pipettes. Open and close all sample tubes and reaction plates carefully to avoid splashing of samples. Briefly spin the tubes/plates before opening to prevent aerosols when opening them.
It is necessary to change gloves from time to time and especially if the gloves get dirty with the specimen or reagent splash. One should not leave the room wearing gloves.
Routine cleaning and disinfecting laboratory
In addition to cleaning and disinfecting the work area before and after every operation with disinfectants such as 10–15% bleach followed by 70% ethanol or turning on the UV lamp of the laminar flow unit for 30 minutes followed by 70% ethanol, a standard procedure must be in place to sterilize the laboratory routinely. There are various disinfection and sterilization options the laboratory can select that will be best suited for its operation.
Following stringent quality control
Laboratory staff should be well trained and follow standard operating procedures, and the laboratory should follow strict quality control procedures. Rate of positive and negative results should be monitored and if a high positive result is observed for a particular analyte, it must be investigated if any cross-contamination has occurred. There needs to be a routine for swipe tests to ensure no environmental contamination has occurred. In the swipe test, a sterile swab dipped in sterile saline is wiped on potentially contaminated surfaces of a laboratory (e.g., benches, pipettes, handles of fridge/freezer, centrifuges, etc.) and then analyzed like a specimen. If the swipe test shows a positive result, it indicates environmental contamination.
Conclusion
As the clinical laboratory working with clinical specimens is prone to contamination, every laboratory professional working in the laboratory should take utmost care in following measures to prevent incidences of cross-contamination. If an episode of cross-contamination occurs, it leads to wasted time, increased costs, and damage to reputation. Hence, it is better to be safe than sorry.
References
1. Press release from the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID 2022. Escmid.org. Accessed March 23, 2023. https://www.escmid.org/fileadmin/src/media/PDFs/2News_Discussions/Press_activities/2022/2022-04-24-Lack_of_glove.pdf.
2. Asgharzadeh M, Ozma MA, Rashedi J, Poor BM, et al. False-Positive Mycobacterium tuberculosis Detection: Ways to Prevent Cross-Contamination. Tuberc Respir Dis (Seoul). 2020;83(3):211-217. doi:10.4046/trd.2019.0087.
3. Cross-contamination of clinical specimens with Bacillus anthracis during a laboratory proficiency test --- Idaho, 2006. Cdc.gov. Published September 12, 2008. Accessed March 23, 2023. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5736a3.htm.
4. OECD. Guidance Document on Good in Vitro Method Practices (GIVIMP). OECD; 2018. https://doi.org/10.1787/9789264304796-8-en.
5. UK Standards for Microbiology Investigations: Good practice when performing molecular amplification assays. Gov.uk. Updated February 19, 2018. Accessed March 23, 2023. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/682533/Q4i5.pdf.
About the Author

Rajasri Chandra, MS, MBA
is a global marketing leader with expertise in managing upstream, downstream, strategic, tactical, traditional, and digital marketing in biotech, in vitro diagnostics, life sciences, and pharmaceutical industries. Raj is an orchestrator of go-to-market strategies driving complete product life cycle from ideation to commercialization.