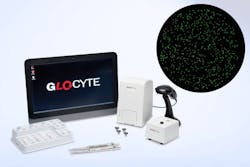
Automated CSF cell counts in 5 minutes
Even a few cells in cerebrospinal fluid can indicate serious conditions such as metastatic cancer or meningitis. GloCyte automates total nucleated cell (TNC) and red blood cell (RBC) counts in under 5 minutes using fluorescence imaging, detecting down to 0 cells/µL and eliminating manual variability.
Arterial and venous blood gas syringes
BD A-line and BD Preset (needleless) syringes preserve sample integrity, minimize preanalytical errors, and improve diagnostic accuracy, designed for collecting whole blood specimens from arterial and venous line draws, empowering critical care decisions. The airtight Hemogard tip cap and self-venting filter of BD Preset expels residual air while maintaining specimen integrity.
COLA expands its accreditation services
COLA's Laboratory Accreditation Program has met the ISO 9001 Standard for quality management since 2013 and applies that same quality focus to the new ISO 15189 Program. COLA's Assessors have an average of 10 years of laboratory management experience and receive extensive annual training. For more details, contact [email protected] and visit cola.org.
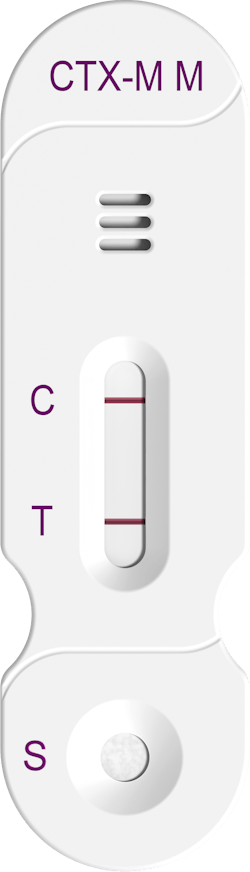
Rapid ESBL detection
Hardy Diagnostics released the new FDA-cleared NG-TEST CTX-M Multi, an in vitro, rapid, and visual immunoassay for the qualitative detection of CTX-M enzymes (groups 1, 2, 8, 9, and 25) for the rapid detection of ESBL’s. Using CTX-M Multi provides detection in just 15 minutes from isolated colonies.
FDA cleared digital cytology system
Hologic’s Genius Digital Diagnostics System is the first and only FDA cleared digital cytology solution, developed to help detect pre-cancerous lesions and cervical cancer cells on a ThinPrep Pap test. The system combines AI and volumetric imaging technology to increase disease detection, enhance screening efficiency, and optimize workflows and resources.
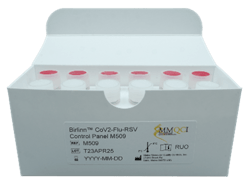
In vitro quality control panel
Birlinn CoV2-Flu-RSV Control Panel M509 is intended for use as a quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of target gene segments in Influenza A, Influenza B, Respiratory Syncytial Virus A/B, and SARS-CoV-2.
Blood gas analyzer with advanced test menu for critical care
Nova’s Stat Profile Prime Plus offers significant clinical value in a blood gas/critical care analyzer by providing unique critical care tests. Blood oxygenation, tissue perfusion status, acid/base balance, electrolyte balance, fluid balance, glycemic control, and kidney function tests are available from two drops of blood in about 90 seconds at the point of care.
Collect, preserve, and transport viral samples
Puritan UniTranz-RT Transport System, with universal transport medium, for the collection, preservation, and transport of viral samples. UniTranz-RT preserves the sample at room temperature and in refrigerated settings. Available with a variety of sterile swabs, including PurFlock Ultra flocked swabs, and as media-only. Visit us in-person at AMP Booth #1129.
High-throughput integrated chemistry and immunoassay system
VITROS XT 7600 Integrated System combines chemistry/immunoassay testing, processes 1,320 tests hourly with MicroTip, MicroWell, MicroSensor technologies and XT MicroSlides. Features Digital Chemistry Optics for precise diagnostics from 2.7 µL samples, 160-test menu, E-CONNECTIVITY Technology and automation options.
*Maximum theoretical throughput. Actual results will vary by test mix and sample workflow.
Streamline your QC, the smart way
The Acusera Smart controls range is designed to fit directly onto a wide range of test systems without the need to aliquot material, streamlining the QC process by minimizing human error and optimizing workflow. For more information visit: https://www.randox.com/acusera-smart-quality-control/.
Upgraded automation for latent tuberculosis testing
The FDA-approved Auto-Pure 2400 liquid handling platform is designed to automate the T-SPOT.TB test, improving laboratories’ productivity while maintaining superior clinical performance in latent tuberculosis detection. The Auto-Pure 2400 combines liquid handling with magnetic cell isolation technology, reduces hands-on time, and delivers accurate, high-throughput TB testing — for in vitro diagnostic use.
Unified, automated, and compliant sample management
Sapio LIMS supports end-to-end sample management for clinical labs, from digital accessioning to final reporting. Features include automated sample lineage tracking, barcode/RFID integration, environmental condition capture, and audit-ready traceability. The no-code platform helps labs enforce SOPs, reduce errors, and maintain compliance with CLIA, GCP, and 21 CFR Part 11.
Ready-to-Use reagent for the quantitative measurement of cholesterol
The SEKISUI Diagnostics SEKURE CHOLESTEROL-SL Reagent is an enzymatic assay that uses cholesterol esterase and cholesterol oxidase as reliable determinants for the quantitative measurement of Cholesterol in serum. This one-part, liquid stable assay comes ready-to-use, saving clinical laboratories time on having to perform additional preparation steps. The SEKURE CHOLESTEROL-SL ASSAY is adaptable for a broad range of clinical chemistry analyzers.
Multiplex LC-MS/MS system
Shimadzu’s Nexera QX incorporates dedicated sample introduction streams for continuous operation of the mass spectrometer, maximizing productivity. The system’s autosamplers, stream-dedicated injection valves and washing pumps deliver ultra-fast performance with ultra-low carryover, while dedicated software offers a single point of control and ensures robust operation for prolonged unattended analysis.
Critical test results wherever they're needed
The epoc Blood Analysis System from Siemens Healthineers is a handheld solution that provides lab-accurate blood gas, electrolyte, and metabolite results at the patient’s bedside or in the field in less than one minute. The single-use Test Card has 13 analytes and requires no refrigeration.
AI-assisted Gram stain analysis
The Fusion Bacteriology Suite: Gram stain solution streamlines bacteriology workflows by digitizing Gram-stained slides. Features include AI-assisted organism detection with automatic calculation of presence, prevalence, and predominance, automated rejection criteria, digital archiving and storage, and an open API for LIS integration.
FDA 510(k)-cleared tacrolimus LC-MS/MS assay
MassTrak Immunosuppressants Kit (IVD) is an FDA 510(k)-cleared LC-MS/MS assay for tacrolimus quantification in whole blood. Used with ACQUITY UPLC I-Class PLUS and Xevo TQD IVD Systems, it provides improved specificity over immunoassay methods. The kit includes chemical reagents, analytical column, calibrators, controls, and validated method instructions.
Molecular controls for HPV testing
ZeptoMetrix NATtrol Human Papillomavirus (HPV) Controls are non-infectious, refrigerator-stable molecular controls formulated with purified, intact whole organisms. Each control contains a target concentration of 1,000,000 copies/mL and is intended for use in molecular assay workflows to support accurate and reliable test results.