Best practices while surviving COVID-19 chaos in the lab
In our second “State of the Industry” survey topic for 2021, Medical Laboratory Observer (MLO) chose to refocus on questions about Best Practices in the clinical lab. With the COVID-19 pandemic influencing the industry, we questioned whether those practices had changed after one year into the pandemic.
Laboratory professionals have had to adjust their best practices to keep up with the changing needs of the pandemic by implementing new workflows and policies, adding new equipment and training to keep quality control in check and revenue flowing. We asked labs how they were dealing with hurdles caused by the pandemic, comparing that data to our Best Practices State of the Industry report from April of 2020, which was sent out in March, just as the pandemic was creeping in, slowing enveloping the world’s laboratories.
How lab directors best maneuver through the unforeseen, while keeping up with the latest products on the market, implementing new training, and all the unexpected challenges of a worldwide crisis is not easy, but here’s insight to keep the lab running optimally. This may include launching a tracking program, opening a new lab, or policy changes, all while keeping up with quality care issues and trying to stay within a reasonable budget.
Running out of essentials
Supply chain issues plagued many labs during the pandemic, and some responded differently than others, with 64% using multiple testing platforms, 57% working with state public health officials to gain access to needed testing supplies, and 45% implementing standing orders for crucial supplies.
Survey responders indicated that 24% of their facilities switched to reusable types of personal protective equipment (PPE), and 20% revamped their product evaluation process, with some paying extreme prices to secure products, using multiple suppliers, doing daily inventory updates system-wide, and using additional areas to store supplies to prevent running out. Others switched brands of products or vendors used, sent specimens out to multiple reference laboratories, and started the ordering process sooner on all items to give an additional couple weeks of lead time.
With supply shortages across the globe, labs had to take extra steps to improve inventory control and supply costs. Nearly 70% evaluated inventory levels for basic supplies, with 45% working with supply chain management for group purchasing organization contracts that offer savings (down from a whopping 73% last year), and 32% used supply tracking and record keeping. Only 9% implemented lease agreements that do not include volume commitments, down from 21% last year, while 10% got access to electronic inventory tracking from materials management, 13% did vendor-managed ordering, and 22% developed an ongoing review, comparing supply reports to invoices.
Walter Valliere, ScD, of Vizient Advisory Solutions, works in analytical labs in biotech, biopharma, research, and those labs serving healthcare, both hospital-based and independent. He analyzed things he could routinely make versus buy and stopped reagent rental agreements to help with supply chain issues; additionally, he implemented new rules to improve efficiency at his facility: no physician-specific test profiles, no standing orders, and no exceptions.
Improving Quality
Striving to continually improve both quality and customer experience, lab workers have taken steps to improve the quality and efficiency of testing, as 65% of survey respondents standardized their test ordering procedures and formularies, 31% programmed hard stops or other functionality in their electronic health records, 23% implemented a pre-approval program for send-out tests, 21% automated manual processes in the pre-analytic phase of testing, but only 7% purchased additional centrifuges to reduce bottlenecks in testing workflows.
To improve the quality of testing last year, 72% said they had standardized ordering procedures to ensure that physicians and other providers order tests correctly, while 23.7% implemented a preapproval program for tests that are sent out, and 27.1% developed evidenced-based test ordering practices.
“Quality is also about the smart use of technology. In recent years, health systems and hospitals have implemented ‘test utilization’ programs to identify patterns in lab ordering,” explained Lee Hilborne, MD, MPH, Senior Corporate Medical Director, Quest Diagnostics. “However, many hospitals use time-consuming manual uploads of data or platforms that cannot pull from disparate enterprise systems.”
Quest introduced a lab stewardship service in 2019 that uses behind-the-scenes technology layered over a health system’s existing LIS and other technologies. The data is shared with doctors and healthcare leaders, so they may compare their practice with medical guidelines, discouraging the use of lower-value or less-proven tests.
“Fundamentally, this helps physicians order the right test for the right patient at the right time. It provides practice feedback regarding situations where tests are inappropriately repeated and improves transparency, providing better patient care. By improving technology and data management, our laboratory pathologists and other laboratory professionals can more easily anticipate needs and help their medical colleagues deliver better care,” said Hilborne.
Adding testing capacity
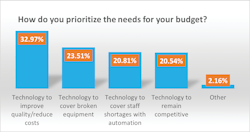
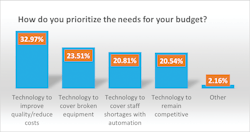
Last year’s top budget priority was technology to remain competitive at 33%, compared with 21% this year. A close second last year, at 32%, was technology to cover broken equipment, which also dropped this year to 24%.
Having so many new analyzers, and software tools, 66% of labs have analyzed workflow processes for proper space planning, which was 70% last year; 54% say they are involving IT early in the process of getting new automation tools, last year was 49%; and 30% have designated a project manager to coordinate planning and implementation with the vendor in getting these new products, with last year being 25%.
“As the pandemic spread, we realized we would need to expand our testing capacity to meet the needs of our patients and clinicians, but also to ensure we would have the capacity due to supply chain challenges,” shared Steven R. McLaren, DO, Assistant Regional Medical Director for the Lab Care Delivery System of Southern California Kaiser Permanente Medical System. Within 70 days, Kaiser Permanente created a COVID-19 lab for all its markets, housing eight Amplitude systems, each capable of 5,000 tests a day, from Thermo Fisher Scientific. They installed a Roche 8800 and 3 Hologic Panthers.
“We also opened up our specimen types to include saliva as well as swab samples. The learning curve with the Amplitudes was steep, and our TAT suffered because of it. It was complicated by the fact we also introduced a new specimen type, which has its own issues requiring attention,” said McLaren. “To round out our services, we have introduced serology testing using the Abbott IgG anti-nucleocapsid assay, and we are assessing the possibility of bringing on-line an anti-spike assay in anticipation of the need for assessing immune status following vaccination.”
Testing and Pandemic Challenges
The pandemic pushed labs to testing limits. The volumes of tests were incredible, with 23% saying they performed 1-2 million tests, 21% performed more than 2 million, another 21% did 100,001-500,000 tests, and 16% did 500,001 – 1 million tests. Only 2% did less than 25,000 tests.
“Where I am working, we commenced testing only after obtaining national accreditation. Additional manpower has been recruited as a temporary measure, which functions for 14 hours a day,” said (Col) Mahendra N Mishra, MBBS, MD (Pathology), ESHI Diploma, of Baptist Christian Hospital in Tezpur, India.
Problems were prevalent during the pandemic, even on the other side of the globe, as Mishra faced a high rate of COVID-19 positivity amongst the staff. “It was the supply of other reagents that impacted us minimally as the workload had declined, and we had to contend with reagents from other vendors who had not supplied us in the past. The hospital has purchased two GenXpert-like (pieces of) equipment for performing cartridge-based testing, and we have a capability of up to 40 tests per day in addition to rapid antigen detection tests.”
With frustrations running high, 77% say they have implemented IT efforts to reduce human error when trying to get reimbursed for testing of non-SARS tests, and nearly 57% have implemented efforts for SARS testing.
“The COVID-19 pandemic has put a tremendous amount of pressure on the already challenged laboratory. It now is more critical than ever for laboratories of all sizes to leverage automation,” said Anthony Barresi, Senior Manager, Beckman Coulter Workflow and IT Solutions.
“For large laboratories that run about 20,000 tests a day, total lab automation is already a reality. However, for medium-volume labs that process fewer than 4,000 tests per day, available solutions are neither total or wholly automated. This is why innovative automation solutions are needed for labs of all sizes - to enable every lab to manage increasing volumes and STAT requests by reducing manual steps, decreasing pre-analytical errors and minimizing turnaround time.”
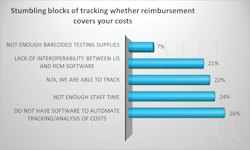
Table 2 illustrates additional steps labs have taken to ensure their costs were reimbursed, with most respondents, 86%, adopting processes to review savings opportunities on a regular basis for non-SARS testing, which was not as readily prevalent for SARS at 38%, likely due to the massive influx of new products designed for COVID-19 on the market at once.
New standard lab processes and staff education materials for SARS were created by 63%, as new education and policies were put into place in response to the pandemic, which may have spilled over into the 79% creating processes and education for non-SARS.
Training lab staff
With new automation comes new training, and when it comes to training the staff on the new software, 60% have created standard workflows for all lab employees, last year was 53%; while 56% created a train-the-trainer model, down from 64% last year. Survey results showed 18% have pulled in the IT department to lead mandatory training for the products, down from 25% in 2020; with 11% doing lunch-and-learn training sessions, way down from last year’s 21%. While 10% of labs have sent a lab person to LIS school to develop an in-house expert for all the new software, which was about half ot last year’s 22%.
Last year, half of the respondents created standard lab processes and staff education materials to help testing run efficiently, and 48% said they adopted analyzers with walkaway testing. Even when things run smoothly, there can be hiccups along the way.Staffing challenges during a pandemic
Costs were not the only hurdle to leap over, as staffing issues became prevalent during the pandemic. Not only did people get sick and get shuffled around to new positions, but staff had to learn new equipment and processes to handle the pandemic, while dealing with supply shortages and other challenges.
A Blood Bank Supervisor and Lab Quality Assurance Coordinator, who asked to remain anonymous, confessed that she ran into chaos during the COVID-19 pandemic. “We ran out of nasopharyngeal swabs, and suppliers could barely keep up with the demand. The manufacturers of our SARS/COVID tests also allowed nasal and throat swabs, so that helped.” She was grateful that her hospital nurses would help collect swabs during day shift.
The facility also faced staffing challenges during the pandemic. When it came to staffing, she explained, “our staff was spread very thin, and several quality assurance items were put on hold nearly the whole year (March to February). No additional staff and no additional pay.” The biggest struggle was scrambling when four lab employees were infected.
Though no new equipment was purchased, they overcame the challenges. “Whenever there were shortages of supplies, our procedures and processes changed, or because of what CDC or the State Health Department recommended that week. There were times when there were new sets of workflow on a daily basis. It got to a point where when we clocked in to work, we had to ask, ‘How are we handling it today?’”
Respondents experienced similar challenges. Hiring freezes were put in place at 38% of labs, while an additional 38% said they saw fear or anxiety among job candidates about working in a lab during the pandemic.
“In early March, as the pandemic was hitting California, the leadership within SCPMG and Kaiser Health Plan, created a Regional Command Center group made up of healthcare providers across multiple disciplines, administrators and ancillary staff. Daily calls were held, which included laboratory leadership,” explained McLaren.
“Out of this support, we were able to stand up internal testing rapidly, which then quickly expanded. Our ability to be at the table with this group of leaders, early in the pandemic, was crucial to our success. The next step was for our lab care delivery system to reach out to the medical center labs to offer support in any way possible. We created a Microsoft Teams chat line, and every other day, we would touch base in group calls with the medical center lab directors that included the lab medical directors. These calls facilitated sharing of best practices, as well as provided an avenue to reach out for help or advice. As the pandemic progressed, the calls did not need to be as frequent and are now held once a week.”Of course, keeping the budget in mind is important. “We look at test volumes and costs, and if we can internalize a test by maintaining or improving on quality, while at the same time decrease our overall costs, we will internalize it. When a test is internalized, it is often done in conjunction with the clinicians or specialty groups to ensure we are going to meet their needs. Often times, we are approached by these same groups who ask for a test to be internalized, because they are experiencing issues with quality or turnaround times.”
Kaiser was not the only company leaping through hoops because of the demands of the pandemic.
“When the pandemic began, we made sure that protecting the health and safety of these employees was our number one priority,” Hilborne said. “We implemented many processes and procedures to foster safe work environments and held special safety training while implementing other key health and safety measures, such as expanded PPE. Many of our employees continue to work long hours to provide 24/7 testing for our patients.”
Staffing challenges hit labs hard during the pandemic. Multiple staff members out sick with COVID-19 at the same time plagued 60% of the labs, while 41% dealt with budget cuts from lost revenue from elective procedures and tests, causing temporary staff furloughs. Making turnaround times difficult to meet, 38% had fewer staff in non-SARS-Cov-2 areas, and 38% had more staff than usual assigned to the pre-analytical area to keep up with SARS-CoV-2 tests.
Policies for social distancing and limiting outsiders in facilities impacted the numbers of internships offered, as there were drops from last year’s best practices survey statistics, as well as staff benefits, such as having an available gym to use as an employee perk.
To retain and recruit, 38% provided continuing education (last year, 60% provided continuing education), 47% offered shift changes or scheduling flexibility, 45% addressed safety concerns, 43% partnered with local colleges and tech schools for internships in their labs, and 42% offered financial incentives, such as sign-on and retention bonuses, up from 36% last year.
Clinical ladders, a structure that encourages professional development from novice to expert, were offered by 34% of lab respondents. Another 33% had daily huddles with peer recognition to boost morale, down from 40% last year, and 21% had a succession-planning process by offering additional responsibilities to their top performers and measuring results, similar to last year’s 21%. Up from last year, 17% lured employees with perks like free parking, reimbursing public transportation costs, an onsite gym or daycare. Perks like these were offered to employees by 16% of labs last year, revealing an uptick in benefits offered to laboratory employees.
“Overall, the pandemic revealed that private and public sector collaboration is critical to addressing any major, rapidly evolving heath crisis. None of us can address major challenges like COVID-19 alone, and none of us can assume that COVID-19 will be the last major pandemic in our lifetimes,” said Hilborne. “This includes the recognition of the value of laboratory professionals to assure the quality of healthcare and the need to assure that our laboratories have sufficiently trained and competent professionals in the decades to come.”
About the Author
Marisa L. Williams
Editor
The author of more than 100 independently published books, Marisa L. Williams earned her Master’s at Johns Hopkins University, while interning on Capitol Hill, doing press and communications for the National Association of Community Health Centers. Creating her own Bachelor of Science degree at the University of Toledo, Williams blended pre-medical, pre-law, and laboratory studies, resulting in an interdisciplinary degree emphasizing Forensics. She has worked with Dr. Paulette Moulton (Levy), a dermatologist in Monroe, MI, as well as Dr. Elizabeth Triana, a family medicine practitioner specializing in hormone therapy, based out of Port Charlotte, FL; additionally, she has 20 years of experience as a multimedia journalist, is a third generation Realtor licensed in MI and FL, and has five years of college level teaching experience.