POC in the lab: a regional experience in urinalysis and pregnancy testing
Horizon Health Network operates 12 hospitals and more than 100 medical facilities, clinics and offices in the province of New Brunswick in Canada. Providing services ranging from acute care to community-based health services, Horizon Health Network has more than 12,400 employees, including 1,000 physicians, and has 5,700 volunteers, auxiliary workers, and alumni. The network consists of four areas with core lab services provided only in the four regional hubs. As part of the provincial laboratory standards, all sites performing point-of-care testing (POCT) must be accredited by the Institute for Quality Management in Healthcare IQMH (former Ontario Laboratory Accreditation 20131). Findings from a 2013 audit identified minor non-conformances specific to ISO 22870 in urinalysis POCT, which remained unresolved during a subsequent audit in 2015 and were escalated to major non-conformances.
Point-of-care (POC) urinalysis testing has been widely evaluated in the literature; studies have demonstrated both the safety and efficacy of both urine test strips and urine hCG testing using semi-automated platforms.2-4 The utilization of POC middleware further facilitates the management of POC testing by providing enhanced traceability of both operators and results—including elements of training, testing patterns, certification, and quality control.5
It was believed that through the adoption of a semi-automated urinalysis/pregnancy testing analyzer, ISO 22870 deficiencies would be promptly addressed. This project demonstrated that in order to deliver optimized patient care and outcomes, laboratory practices and quality processes need to be incorporated into the technology implementation methodology.
Materials and methods
A technology review utilizing a Request for Information (RFI) in accordance with provincial procurement guidelines was conducted to identify vendors able to address the defined clinical criteria for a regional urinalysis POCT solution. Requirements identified were on-board quality control lockout, operator management, barcode scanning capabilities, and, ideally, a diverse test menu. A vendor was selected to provide the middleware and workflow consulting services.
A pre-implementation needs assessment was conducted with each of four regional POC coordinators to identify current state and key needs/gaps. Findings were shared with the team as part of a change management seminar.
The implementation plan for both instruments and software was designed with users to adhere to quality standards, and each analyzer was validated at the regional center prior to being distributed to the POC site/location. Training was provided via on-site workshops utilizing a ‘train the trainer’ format. Additional on-line training was available through the vendor for users.
A post-implementation survey was conducted in July 2016 via Survey Monkey, sent to 466 end users. Additional analytical data was downloaded from the server. Both the survey results and data identified gaps in preliminary implementation; workflow mapping was utilized to identify root causes of identified gaps. Corrective actions were taken including some retraining, adjustments to some instrument settings, and workflow modifications. Data was downloaded to look at all of 2016.
Overall success of the project was measured using internal audits post-installation and an external audit (IQMH laboratory accreditation) in February-April 2017 to successfully demonstrate adherence to ISO 15189 Plus standards (ISO 22870 requirements not previously met).
Results
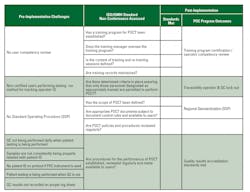
IQMH audit findings between 2013 and 2015 emphasized nine minor non-conformances to ISO 22870:2006, which could not be resolved by current processes and technologies for POC. Pre-implementation survey responses were received from all four regional sites with the opportunity to identify current state challenges that needed to be addressed. Eight key findings are outlined in Table 1, emphasizing the lack of a standard operating procedure (SOP), including no consistent quality control (QC) or traceability.
The implementation phase included a “train the trainer” program that covered change management as well as technical and product training. Questions on the training and implementation process were captured in the post-implementation survey.
Following the implementation, three independent methods were used to demonstrate the effectiveness of the results:
Post-implementation survey. Survey results were received from 74 respondents (16 percent), with the distribution of respondents reflective of the instrument distribution across the regions. Training provided by the regional POC trainers was identified as a strength of the implementation process. User feedback highlighted general satisfaction around the program. Positive comments were made about instrument ease-of-use, increased confidence in quality of patient results, and reporting process overall. Some key lessons around the need for education on quality (laboratory) standards as well as increased communication and change management will be included in the discussion section.
Instrument utilization data. Instrument utilization was collected by instrument and by site utilizing the data management software to evaluate test utilization and QC compliance. Data was collected in September 2016, and again in December 2016. First data analysis identified higher than expected QC frequencies in some locations compared to the implemented SOP, and the workflow analysis uncovered instrument settings that were not set in accordance with the once-daily QC requirement. The December 2016 review of data demonstrated that the QC issue was corrected and highlighted an increased utilization of tests across the majority of sites. The ability to easily access data has enabled the regional POC coordinators to benchmark results on an ongoing basis to identify any differences in practice and address potential variance in an efficient and coordinated way.
Spot and formal audit findings post-implementation. Spot audits were conducted at each of the Moncton Area, New Brunswick, sites with additional audits scheduled to be completed on an ongoing basis in each of the regions. Of note, the audits to date identified 100 percent compliance with patient identification and only one minor issue (results unsigned), which was immediately traced back to the end user and addressed.
Conformance results included in Table 1 highlight the process improvements made from the preliminary audits through today.
Discussion
POC testing continues to expand, with many sites undergoing similar challenges adhering to increasing rigorous local requirements, including ISO standards. While every clinical situation is unique, there are some key lessons that can be readily applied by our colleagues across Canada and around the world.
Extensive global experience with new technology implementations in core labs provides evidence that new technology alone is not sufficient to achieve objectives. Workflow assessment and process redesign are required to optimize outcomes that are possible with new technology. Holding onto old processes when implementing new technology in any diagnostic pathway, including POC, limits the success of the program. Process redesign, regardless of where it occurs, requires thought and active management. In the laboratory environment, the core stages are common; however, survey results and onsite workflow assessment post-implementation highlight the importance of a different style of communication when implementing new processes in a POC program.
Working with other healthcare professionals is quite different than working with lab professionals. A laboratorian inherently understands the need for rigor and structure around quality management. As POCT expands to non-laboratorians, quality management can be perceived as a nuisance or barrier to providing immediate care. Feedback received from end users readily acknowledged that quality improved, but the process change was challenged due to the perceived delays/incremental steps that are required as part of a quality testing process.
In developing training programs for new technology implementation and processes, it is equally critical to explain the “why,” or need for certain standards to be in place, as the “how”—the way things need to be done. Utilizing Kotter’s change management model7 as a foundation, the development of a change management plan and related practices is critical to quick adoption of new technology and compliance to SOPs. Feedback from end users and onsite workflow assessments highlighted the need for a structured change management plan in POC implementations. Change management practices will answer questions such as “Why do I need to run Quality Control?” and provide education for non-laboratorians on how new POC testing process steps are important to patient safety and ISO standard requirements.
The adoption of ISO standards to expanded laboratory services in the POC setting is an opportunity to bring lab standards to all aspects of patient care. In order to maximize the program’s success and consequently have a positive impact on patient outcomes, it is critical to combine the right technologies (instruments, assays, middleware) with optimized processes and robust training that includes change management practices.
REFERENCES
- Ontario Medical Association. Ontario Laboratory Accreditation (OLA) Requirements Version 6, Dec 2013.
- Tighe P. Urine dry reagent strip ‘error’ rates using different reading methods. Accred Qual Assur 2000;5:488-490.
- Milhorn DM, Korpi-Steiner NL. Evaluation of Clinitest hCG device susceptibility to high-dose hook effect by intact human chorionic gonadotropin and hCG beta-core fragment at concentrations observed in early natural pregnancy. Clin Chem. 2014;60:s217 B-297.
- Crocker JB, Lee-Lewandrowski E, Lewandrowski N, Baron J, Gregory K, Lewandrowski K. Implementation of point-of-care testing in an ambulatory practice of an academic medical center. Am J Clin Pathol. 2014;142:640-46.
- Gramz J, Koerte P, Stein D. Managing the challenges in point of care testing. Point of care. 2013;12:76-79.
- Siemens Healthineers. Siemens healthcare consulting solutions change management guide. 2015.
- Kotter J, Cohen, D. The Heart of Change: Real life stories of how people change their organizations. Harvard Business School Press. Boston, MA. 2002.