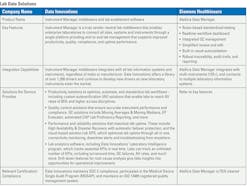
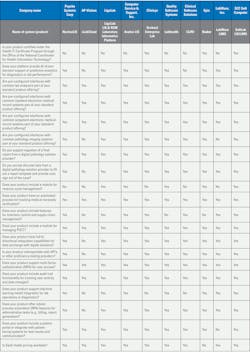
Technology advances in the lab: MLO’s 2025 LIS and Lab Data Solutions Guide
Medical Laboratory Observer (MLO) publishes a Laboratory Information Systems (LIS) Buyer’s Guide annually. We started including lab data products from companies outside the LIS in 2024, and this year are officially introducing the MLO LIS and Lab Data Solutions Guide.
In addition to the product tables, we interviewed seven laboratory technology experts who gave their insights on current and future technological developments in the clinical laboratory, suggestions on how vendors can help ease the process of adopting LIS and other technologies in the lab, advice for lab leaders, and the evolution of LIS.
Technological advancements
The current biggest technological advancements impacting LIS and other lab data tools in 2025 are artificial intelligence (AI), cloud-based products, and interoperability, according to the experts. Rob Brown, Head of the Scientific Office, Sapio Sciences emphasized, “The most transformative advancements impacting lab information tools are focused on intelligence, usability, and configurability. Lab information tools are moving beyond passive record-keeping to integrate AI that acts as a co-scientist, helping with experiment design, result analysis, and the recommendation of next steps.”
“Voice is also emerging as a new interface in both research and diagnostics labs, allowing scientists to interact with their LIMS using natural language. These advancements are supported by unified platforms that connect various tools in a single environment, reducing handoff errors and enabling full traceability,” he said.
Suren Avunjian, CEO, LigoLab observed the quick adaptation and flexibility of laboratories as new technologies are rolled out. “Automation and AI are no longer experimental; they’re embedded into modern LIS platforms. Labs are utilizing agentic tools to automatically flag inconsistencies, interpret results, apply AI to digital slides, scan paper requisitions and import them as an order, voice-to-text and command control of the LIS, and generate customized reports. This reduces manual review and allows staff to focus on higher-value clinical work,” he said.
Kim Futrell Senior Strategic Marketing Manager, Clinisys said, “In 2025, cloud-based LIS platforms have become foundational to modern lab operations, offering remote access, scalability, and built-in disaster recovery. With rising concerns around data privacy, vendors are enhancing cybersecurity and compliance features to meet regulatory demands. A primary emphasis is placed on interoperability, with LIS platforms increasingly engineered to seamlessly connect with EHRs, digital pathology, genomics, and AI diagnostics.
She added, “Structured data frameworks facilitate seamless integration, allowing labs to function within broader healthcare ecosystems. Utilizing embedded analytics tools powered by pay-per-use cloud computing, labs can access real-time dashboards and insights into test utilization, staffing, and patient outcomes—optimizing performance and demonstrating value. Simultaneously, digital pathology and AI are revolutionizing remote collaboration, accelerating image analysis, and creating adaptive workflows that cater to evolving diagnostic needs. Moreover, AI presents innovative opportunities for enhancing service and support delivery models.”
Avunjian continued, “With open APIs and interoperability standards, LIS systems are now able to connect seamlessly with instruments, EHRs, billing platforms, and digital pathology solutions. This creates a more unified ecosystem and reduces the silos that have traditionally hindered lab efficiency and growth.” He noted that with all this, labs are also focusing more on cybersecurity, complying with regulations, and hardening their systems against ransomware and hacker attacks. “Modern systems incorporate embedded encryption, robust audit trails, and predictive analytics, enabling labs to reduce both risk and waste across a range of applications, from instrument maintenance to reagent management,” he said.
Furthermore, “All of these advancements are converging into comprehensive informatics platforms, systems of action that cover the entire lifecycle of a case, from accessioning through revenue capture,” according to Avunjian.
Integration hurdles
When asked what challenges labs face when adopting or upgrading LIS and other data platforms, Gilbert Halkim, CEO, SCC Soft Computer mentioned mindset as the main obstacle. “Too often, labs try to replicate their legacy workflows in a new LIS. The best results come when labs adopt vendor best practices, streamline implementations, and leverage modern functionality without over-customization.”
Labs often face significant challenges from legacy systems that create rigid workflows, siloed data, and a frustrating user experience, Brown said. “These issues directly impact critical metrics like turnaround time, sample traceability, and regulatory compliance. A key problem often occurs in the pre-analytical phase, where manual data entry and poor sample handoffs introduce errors.”
Eric Dingfelder, President and CEO, Data Innovations pointed out another barrier. “One of the most significant challenges laboratories encounter when upgrading their Laboratory Information Systems (LIS) or other data platforms is achieving seamless interoperability across diverse systems, instruments, and automation technologies. Integrating these components—often from different vendors—can be complex, time-consuming, and costly,” he said.
Futrell said, “Staffing shortages often limit the time and resources available for implementation and training, delaying adoption, curtailing new feature adoption rates and reducing onboarding effectiveness. Labs are required to maintain fast turnaround times and avoid any disruptions that could impact result delivery and provider satisfaction. Additionally, they must ensure compliance with HIPAA, CLIA, and FDA standards, including secure data transmission and audit trails. It is essential that LIS platforms connect seamlessly with lab instruments and external systems to prevent workflow disruptions.”
How vendors can help
Vendors can help ease the process of adopting LIS and other technologies in the lab by guiding labs toward proven models while still aligning with their operational goals, according to Halkim.
Brown suggested vendors offer modern platforms with out-of-the-box solutions designed for real-world lab workflows. “Configurability is essential, as it allows labs to adapt processes without relying on developers. Additionally, intuitive interfaces, including voice and natural language tools, simplify onboarding and help resolve key bottlenecks: TAT, traceability, data fragmentation, and compliance,” he said.
Futrell added, “Vendors play a crucial role in mitigating these challenges. By offering flexible and scalable solutions, they allow labs to adopt new systems gradually, minimizing disruption. Robust implementation support—including project management, training, and technical assistance—can ease the burden on lab staff. Advanced connectivity tools help eliminate middleware and ensure smooth communication between lab instruments and third-party systems.”
Dingfelder pointed to his company's solutions when asked how vendors can help ease the integration burdens on labs. “Data Innovations offers vendor-neutral middleware solutions that simplify connectivity and data exchange. Instrument Manager middleware enables centralized integration and oversight of unlimited LIS platforms, instruments, and in vitro diagnostic devices. This allows labs to unify operations across multiple disciplines, locations, and systems—enhancing efficiency, scalability, and data consistency.”
Advice for lab leaders
Labs should look beyond current features and evaluate a vendor’s roadmap for AI, interoperability, and patient-facing tools, said Ed Price, CEO, Computer Service & Support, Inc. “Just as important, they should ensure they own and can access their data. Too many systems restrict exports or lock customers into closed databases. Avalon gives clients direct read-access, empowering them to build their own analytics, integrate external tools, and retain full control. The LIS platforms that balance compliance, innovation, and empowerment will deliver the greatest long-term value.”
Jomah Williams, Product Manager for IT, Siemens Healthineers emphasized the importance of choosing a solution that will evolve with the laboratory. “Within any cost associated purchase, you want value and a vehicle that can handle the growth of your lab needs as well as offering innovate for the functions.”
Halkim offered clear advice, “Don’t compromise by cutting corners. Implementing systems without investing in a robust LIS can create long-term limitations. Instead, partner with a state-of-the-art LIS that supports advanced interoperability, scalability, and modern lab workflows. This ensures labs remain agile, secure, and future-ready.”
A common observation is that a neutral platform allows for adaptation to changes in the LIS or device manufacturer without being limited by operational tools that may not align with evolving laboratory needs, Dingfelder added. “It is advisable to consider flexibility and adaptability in both purchasing and change management decisions when selecting a partner.”
The evolving LIS
LIS platforms are required to evolve to support interoperability with EHRs, digital pathology, genomics tools, or AI diagnostics.
“Interoperability is now essential,” Price stressed. “Avalon LIS supports HL7, FHIR, and modern APIs to connect directly with major EHRs. We’re expanding these capabilities to integrate with digital pathology systems, genomics data, and external AI diagnostics - enabling true bi-directional data exchange rather than isolated workflows.”
Williams said, “Auto-verification workflows drive faster results and integration with many new devices like middleware providers and real time data streaming. Standardization of result protocols leads to consistent quality.”
The stress on laboratories to use more technologies and the need for one seamless workflow for EHRs, digital pathology tools, genomics data, RCM, and AI diagnostics was spotlighted by Avunjian. “Advanced LIS platforms are evolving to make this possible by serving as the central hub where all of these technologies come together.
Digital pathology is a prime example. Moving from glass slides to high-resolution digital images allows pathologists to review cases remotely, collaborate with peers, and use AI to highlight patterns that the human eye may miss or fill out the report with the AI diagnosis in preparation for the pathologist to review and sign out. But scanners and viewers on their own do not solve the interoperability challenge. That requires a modern LIS.”
He continued, “Diagnostic accuracy improves when AI and human input are combined in a unified report. Turnaround times drop because case data, images, and results are all in one place. Collaboration becomes easier, whether within a hospital network or across the globe. Most importantly, patient care improves because clinicians receive complete, timely, and clear results. By building interoperability into the core of an advanced LIS platform, labs are provided with the foundation to adapt to the digital future.”
The next frontier
We asked the experts, ‘What do you see as the next frontier in lab data management and analytics? Are there any untapped opportunities labs should be preparing for now?’ Here is what they said:
Suren Avunjian, CEO, LigoLab
The next frontier in lab data management is real-time intelligence. Instead of waiting for daily reports, labs will run on live streams from instruments, couriers, portals, and payers. Systems will predict backlogs, reagent shortages, and staffing needs before they happen. To get ready, labs should standardize specimen, order, and patient IDs and define one source of truth for events.
Another big step is bringing multiple data types together. Images, genomics, proteomics, and EHR data will need to connect at the case level. This requires clean metadata. Labs should start enforcing consistent accession keys, site codes, and ontologies now and assign ownership for maintaining them.
Patients will expect results that are easy to read with clear next steps. Clinicians will want traceable logic. Labs should prepare by storing report provenance, rule versions, and QC evidence so every conclusion can be backed up.
Revenue integrity will also move into real-time. Variances, denials, and eligibility gaps will be flagged the day they occur. Labs can prepare by normalizing payer names, contract terms, and denial codes. Feedback loops should link denials back to order capture and coding rules.
Privacy-preserving model training will become routine. Federated learning will allow cross-lab insights without exposing PHI. Labs should classify data, document consent, isolate PHI fields, and capture audit trails for all model inputs and outputs.
Digital pathology will scale up. Image analytics will handle triage, QC, and measurement extraction automatically. Labs need to standardize scanner metadata, slide IDs, magnification, and stain labels, while also validating storage and retrieval speeds.
Data contracts will govern interoperability. APIs will be versioned and tested against set standards. Labs should treat HL7 and FHIR as managed products and retire custom transforms.
Operational twins will model staff, instruments, and workflows so leaders can test changes before implementing them. Labs should start capturing cycle times by step and shift in a form that supports simulation.
There are also untapped opportunities. Labs can link turnaround times to patient outcomes like length of stay or therapy start dates. Outreach data can reveal new service lines. Benchmarks can be published for clients. Advisories can be offered when trends change.
The first step is governance, clean identifiers, and event streams. Then build dashboards that drive daily action. Advanced models will add value only if the foundation is solid.
Eric Dingfelder. President and CEO, Data Innovations
As AI applications become more pervasive, we are seeing the shift away from exception-based dashboards to opportunity-based intelligence generated by AI tools in support of data lakes. Lab staff should prepare themselves for not just utilizing tools but prepare for AI as a peer especially in areas like AI-powered decision support in staffing, test utilization, and operational planning.
Kim Futrell Senior Strategic Marketing Manager, Clinisys
The next frontier lies in predictive analytics and personalized diagnostics. As labs accumulate more longitudinal data, there is an opportunity to use machine learning to anticipate testing needs, detect emerging health trends, and tailor diagnostics to individual patients. Labs should also prepare for increased collaboration with public health agencies and research institutions, as data sharing becomes more critical in global health initiatives.
Gilbert Halkim, CEO, SCC Soft Computer
The next frontier is AI-driven automation of result interpretation and release. This represents a shift from support tools to active decision-making partners. Labs that prepare now will position themselves to leverage AI not just for efficiency, but also for enhanced diagnostic accuracy and scalability.
Ed Price, CEO, Computer Service & Support, Inc.
The next frontier is predictive operations and proactive care. By analyzing test volumes, turnaround times, and population health data, LIS platforms will forecast demand, optimize staffing, and anticipate trends. Labs that prepare now will shift from reactive service providers to proactive partners in patient care.
Jomah Williams, Product Manager for IT, Siemens Healthineers
Virtual environment and cloud-based informatic eco-systems, as well as cybersecurity protocols. Connectivity of the middleware to multiple instruments from other OEMs.