Improved detection of acute Lyme disease with MTTT
Earning CEUs
For a printable version of the March CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
MARCH LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Recall the causative agent of Lyme disease and estimated average of cases per year.
2. Describe the need and development of Lyme disease testing algorithms.
3. Discuss limitations of the STTT testing algorithm for the detection of Lyme disease.
4. Discuss the validation study findings of MTTT testing algorithms and their benefit to earlier developed algorithms.
Lyme disease is the most common vector-borne disease in the United States. In North America, it is transmitted to humans by the bite of an Ixodes spp. tick, which is harboring and subsequently passes the spirochete Borrelia burgdorferi. During the 10-year period of 2008-2018, the Centers for Disease Control and Prevention (CDC) reported an average of more than 26,000 confirmed cases annually of Lyme disease in the U.S. Within that same period, the CDC estimated that an average of greater than 9,000 probable cases of Lyme disease were not reported, bringing the number of total cases of Lyme disease in the U.S. to over 35,000 cases per year.1
While 35,000 cases per year is significant, this number is likely drastically underestimated. Using medical claims data to estimate the number of possible cases of Lyme disease, in 2015, the CDC estimated that greater than 300,000 cases of Lyme disease occur each year in the U.S.,2 which is more than 10 times the number of cases reported. This all translates into more than 3.3 million laboratory tests for Lyme disease performed annually.3
Initial test options – IFA and ELISA
In 1982, when the causative agent of Lyme disease was determined, a whole host of tests were developed to aid in the diagnosis of Lyme disease. The first test approved by the FDA for commercial distribution was a Borrelia burgdorferi indirect fluorescent assay (IFA) that was cleared in 1987, which is still in use to this day.5 The following year, a number of ELISA tests for measuring antibody to Borrelia burgdorferi became commercially available. ELISA tests were, and still are more widely used since they are easily automated and provide an objective result. Despite the simplicity and objectivity of those ELISA tests, the lack of agreement among commercial products prompted the need for guidance.
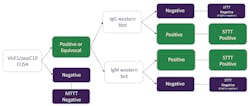
In 1994, several sponsors, including the Association of Public Health Laboratories (APHL), the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH) and the U.S. Food and Drug Administration (FDA) held a meeting in Dearborn, MI, called the Second National Conference on Serologic Diagnosis of Lyme Disease.4 A portion of the conference was devoted to devising recommendations for serologic test performance and interpretation. The outcome was a two-test serologic approach for detecting active disease and for previous infection that has come to be known as the Standard Two-Tiered Testing (STTT) algorithm (The STTT paradigm is depicted in Figure 1).
MTTT challenges the standard
The STTT algorithm typically incorporates an ELISA as the initial screening test. Any specimens that are positive or equivocal on that initial screen are subsequently tested by an IgG and/or an IgM immunoblot. For over two decades, the STTT algorithm has been the standard for Lyme disease serology despite documented shortcomings.6,7,8 The most significant shortcomings are that many first-tier ELISA tests lacked specificity resulting in an excessive number of unnecessary second-tier immunoblots, which seemed to be even more problematic.
Most notably, the Western blots seemed to be less sensitive in early disease compared to the first-tier ELISA tests leading to possible STTT false negatives. The IgM Western blots had relatively poor specificity leading to potential STTT false positives. Finally, second-tier Western blots employed a subjective interpretation and were technically challenging to perform. With more than 3.3 million Lyme disease tests performed in the U.S. each year, a growing global prevalence of this elusive disease, and the STTT testing limitations noted above, there was a need for alternative diagnostic serology algorithms that could improve detection of early disease, while maintaining similar specificity to the STTT algorithm.
In 2003, Bacon et al. demonstrated that the combination of certain second-generation ELISA tests – some commercial and some non-commercial – showed improved sensitivity in early disease as compared to STTT.6 In a commentary letter related to that publication,9 it was suggested that a combination of first-tier, commercial ELISA tests should be considered as an alternative for STTT. These were some of the early indicators that running specimens on a combination of first-tier screening tests might be more sensitive, yet equally specific as the STTT algorithm. Using a combination of commercially available, FDA-cleared Borrelia screening assays, it was demonstrated that using these sequential, first-tier screening assays showed superior sensitivity in early Lyme disease while keeping specificity constant.10
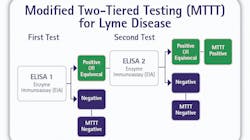
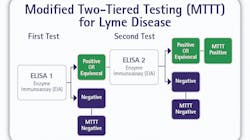
This study employed a Borrelia whole cell lysate ELISA followed by a VlsE1 and pepC10 multiplex immunoassay. This investigation revealed that replacing the Western blot with a multiplex immunoassay resulted in a 20.7 percent increase in sensitivity while maintaining specificity at 95.6 percent. Finally, Branda et al. showed that using a modified two-tiered testing (MTTT) algorithm comprised of two FDA-cleared, first-tier screening ELISA tests, resulted in significantly improved sensitivity for the detection of early disease while maintaining comparable specificity.11 This laid the groundwork for a series of similar STTT versus MTTT studies that have been previously summarized.12 All such studies seem to convey a common theme; two sequential first-tier ELISA tests have the potential to provide improved sensitivity in the detection of early Lyme disease, while providing comparable specificity (The MTTT algorithm is depicted in Figure 2).
ELISA and the MTTT algorithm
Published studies clearly demonstrated the potential advantages of the MTTT algorithm, which are improved serodiagnosis of early cases of Lyme disease and the removal of the technically challenging and subjective immunoblots. The problem was that although the scientific community had adequately demonstrated over a period of many years that the “two ELISA” MTTT concept was clearly comparable, if not superior to the decades old STTT paradigm, without FDA clearance and CDC endorsement of the MTTT algorithm, there was little chance that clinical laboratories could take advantage of the MTTT concept. Likewise, without the lab’s ability to fully adopt MTTT, it was inevitable that many infected patients with early Lyme disease who may actually be seropositive with the MTTT algorithm, may yield seronegative results with the STTT algorithm.
A new investigation to validate the MTTT algorithm was envisioned that incorporated four different FDA-cleared anti-Borrelia ELISA tests. If successful, this would allow for the MTTT concept to be used as an option for clinical labs. The four tests included one whole cell lysate ELISA that detected both IgG and IgM class antibodies, one whole cell lysate ELISA that detected IgG only, one whole cell lysate that detected IgM only and one second-generation IgG/IgM ELISA that incorporated a mixture of recombinant VlsE1 and synthetic pepC10 antigens.
With input from the FDA, an IRB-approved, investigational protocol was outlined that would empirically demonstrate if an all-ELISA, MTTT concept could serve as a substantially equivalent alternative to STTT. The general study design would test specimens with a first-tier ELISA test. Then, as per the STTT algorithm, any specimens that were positive or equivocal on the screen test would be reflexed to FDA-cleared Western blots. Those same samples were also tested on a second ELISA test. With that basic design, a direct comparison could be made with the Western blot as the confirmatory test versus the second ELISA as the confirmatory test.
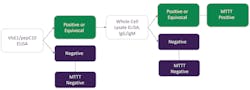
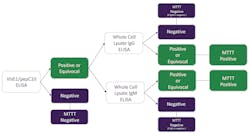
It has been previously pointed out that the most widely published concept for MTTT was to initially screen with an ELISA test comprised of a whole cell lysate antigen, followed by an ELISA test comprised of a second-generation (recombinant and/or synthetic) antigen preparation.7 However, it is also known that alternative scenarios have been validated and perform equally well. In this study, specimens were screened using the second-generation ELISA comprised of VlsE1/pepC10 antigen and confirmed using whole cell lysate ELISA(s). This scenario, coupled with three FDA-cleared whole cell lysate ELISA tests, enabled the investigators to envision two separate MTTT concepts (Those two MTTT concepts are depicted in Figures 3 and 4).
MTTT-1 and MTTT-2 algorithms
With the MTTT-1 algorithm, specimens negative on the VlsE1/pepC10 IgG/IgM ELISA were considered both STTT and MTTT-1 negative. Specimens positive or equivocal on the VlsE1/pepC10 IgG/IgM ELISA were reflexed to IgG and IgM Western blot and the Borrelia whole cell lysate IgG/IgM ELISA. With respect to STTT second-tier analysis, specimens positive by IgG and/or IgM Western blot as per the CDC guidelines for interpretation of Western blot4 were scored as STTT positive. Specimens that were negative for both the IgG and IgM Western blot were scored as STTT negative. For the MTTT-1 second-tier analysis, specimenspositive or equivocal for the Borrelia whole cell lysate IgG/IgM ELISA were scored as MTTT-1 positive, and those that were negative on the second-tier ELISA were scored as MTTT-1 negative.
The MTTT-2 algorithm was similar except that if the specimens were positive on the first-tier screen ELISA, in addition to being reflexed to IgG and IgM Western blot, they were reflexed to two separate second-tier ELISA tests; one was a Borrelia whole cell lysate IgG ELISA and the other was a whole cell lysate IgM ELISA. In this case, the MTTT second-tier ELISA tests were treated much like the conventional Western blot in the STTT protocol. If either of the second-tier ELISA tests were positive or equivocal, the specimen was considered MTTT-2 positive, and if both of the second-tier ELISA tests were negative, the specimen was scored as MTTT-2 negative (The STTT algorithm utilized is outlined in Figure 5).
With this experimental design, the FDA was provided with important data showing how MTTT compared to STTT using two specific patient cohorts; one comprised of extremely well-characterized clinical specimens and one comprised of prospectively collected, routine submits representative of samples typically analyzed by the average clinical lab. More specifically, the FDA was provided with data comparing MTTT-1 compared to STTT and MTTT-2 compared to STTT utilizing four different ELISA products which had been previously cleared for commercial use. These data enabled results to be shared relative to clinical outcome, as well as results relative to typical STTT performance.
For the prospective cohort, comparing MTTT-1 to STTT on the 2,932 specimens resulted in relative sensitivity, relative specificity and relative agreement of 99 percent, 98 percent and 98 percent respectively. When the same cohort was evaluated using the MTTT-2 algorithm compared to STTT, the relative sensitivity, relative specificity and relative agreement were 100 percent, 96 percent and 97 percent respectively. Both the MTTT-1 and MTTT-2 algorithms demonstrated a high degree of correlation relative to the existing STTT (depicted in Figure 5). These data are consistent with the many published studies comparing STTT to various MTTT algorithms.
Using the retrospectively collected, clinically characterized cohort allowed the FDA to review data summarizing clinical sensitivity and clinical specificity of all three algorithms: STTT, MTTT-1 and MTTT-2. In the non-Lyme disease control group, the clinical specificity of STTT, MTTT-1 and MTTT-2 were 100 percent, 99 percent and 99 percent, respectively. In both MTTT scenarios, there were two patients diagnosed with mononucleosis that were MTTT positive and STTT negative.
In the clinically characterized, Lyme disease cases, the clinical sensitivity of STTT, MTTT-1 and MTTT-2 were 73 percent, 81 percent and 89 percent respectively. In both MTTT scenarios, the clinical sensitivity was significantly greater than the traditional STTT paradigm. As has been published previously, STTT and MTTT compared well in late, stage 3 Lyme disease; however, in early disease, especially acute stage 1 Lyme disease, the sensitivity of MTTT compared to STTT was significantly better. Specifically, in acute stage 1 Lyme disease, MTTT-2 detected 27 percent more cases of disease than STTT. While slightly fewer, MTTT-1 detected 23 percent more cases of early Lyme disease compared to STTT.
FDA clearance leads to paradigm shift
The FDA submission confirmed what nearly a decade of various scientific studies had documented; using multiple, sequential, tier-one Borrelia screening ELISA tests can significantly improve the detection of early Lyme disease compared to the 1994 STTT algorithm. In some cases, clinical sensitivity can be improved by as much as nearly 30 percent. Considering that the CDC estimates that there are approximately 300,000 cases of Lyme disease in the U.S. annually, there are likely tens of thousands of infected patients per year who have been tested for Lyme disease using the traditional STTT method and found to be seronegative; that would have been seropositive if tested by the MTTT-1 or MTTT-2 algorithms.
Considering the likelihood of improved patient outcomes, and the substantial equivalence that was demonstrated, the FDA cleared the two algorithms in July of 2019.13 Shortly thereafter, the CDC subsequently modified their recommendations regarding Borrelia serology,14,15 which represents a major paradigm shift as it relates to the 25-year-old STTT algorithm. A shift that will undoubtedly lead to greater operational efficiency in clinical laboratories and improved serodiagnosis of early Lyme disease.
For a printable version of the March CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
REFERENCES:
- Lyme Disease Charts and Figures; Historical Data. Centers for Disease Control and Prevention website https://www.cdc.gov/lyme/stats/graphs.html Updated November 22, 2019. Accessed January 20, 2020.
- Nelson CA, Saha S, Kugeler KJ, et al. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerging Infectious Diseases. 2015;21(9):1625-1631. doi:10.3201/eid2109.150417.
- Hinckley AF, Connally NP, Meek JI, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59(5):676–681. doi:10.1093/cid/ciu397
- Recommendations for Test Performance and Interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Centers for Disease Control and Prevention Website https://www.cdc.gov/mmwr/preview/mmwrhtml/00038469.htm Updated November 19,1998. Accessed January 20, 2020
- 510(k) Premarket Notification Database. IFA test for antibodies to Borrelia burgdorferi
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K871246 Updated January 13, 2020. Accessed January 20, 2020. - Bacon RM, Biggerstaff BJ, Schriefer ME, et al. Serodiagnosis of Lyme disease by kinetic enzyme- linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis. 2003; 187:1187–99. doi.org/10.1086/374395
- Branda JA, Body BA, Boyle J, et al. Advances in Serodiagnostic Testing for Lyme Disease Are at Hand. Clin Infect Dis. 2018;66(7):1133–1139. doi:10.1093/cid/cix943
- Moore A, Nelson CA, Molins C, et al. Current Guidelines, Common Clinical Pitfalls, and Future Directions for Laboratory Diagnosis of Lyme Disease, United States. Emerging Infectious Diseases. 2016;22(7):1169-1177. doi:10.3201/eid2207.151694
- Porwancher RB, Cost-Effectiveness of Peptide-Antigen Immunoassays for Lyme Disease, J Infect Dis. 2004; 189(10):1962, https://doi.org/10.1086/383480
- Porwancher RB, Hagerty CG, Fan J, et al. Multiplex immunoassay for Lyme disease using VlsE1-IgG and pepC10-IgM antibodies: improving test performance through bioinformatics. Clin Vaccine Immunol. 2011;18(5):851–859. doi:10.1128/CVI.00409-10
- Branda JA, Linskey K, Kim YA, et al. Two-Tiered Antibody Testing for Lyme Disease with Use of 2 Enzyme Immunoassays, a Whole-Cell Sonicate Enzyme Immunoassay Followed by a VlsE C6 Peptide Enzyme Immunoassay, Clin Infect Dis. 2011; 53(6):541–547, https://doi.org/10.1093/cid/cir464
- Marques AR. Revisiting the Lyme Disease Serodiagnostic Algorithm: the Momentum Gathers. J Clin Microbiol. 2018;56(8):e00749-18. Published 2018 Jul 26. doi:10.1128/JCM.00749-18
- FDA clears new indications for existing Lyme disease tests that may help streamline diagnosis. U.S. Food and Drug Administration website https://www.fda.gov/news-events/press-announcements/fda-clears-new-indications-existing-lyme-disease-tests-may-help-streamline-diagnoses Updated July 29, 2019. Accessed January 23, 2020.
- Mead P, Petersen J, Hinckley A. Updated CDC Recommendation for Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 2019;68:703 http://dx.doi.org/10.15585/mmwr.mm6832a4
- CDC Supports Development of New Tests. Centers for Disease Control and Prevention website. https://www.cdc.gov/lyme/diagnosistesting/index.html Updated November 20,2019. Accessed January 23, 2020.
About the Author

Mark Kopnitsky, B.S., M.S
With more than three decades experience in the development and commercialization of IVD products, Mark has submitted and cleared more than 120 IVD products through the Food and Drug Administration using the 510(k)/PMA process. At ZEUS Scientific, he provides technical support to all areas of the company, and oversees the Research & Development, Quality Systems, Regulatory Affairs and QA/QC departments. ZEUS’ current product line of IVD includes nearly 200 tests for various autoimmune disorders and infectious diseases.