Editor’s note: Frank Wians, PhD, raises an issue about the February 2016 Continuing Education article “Understanding the CDC’s updated HIV test protocol” (MLO. 2016;48(2):8-13). Here is Dr. Wians’ letter, followed by a response from the article’s author, Robert Kapler.
The article, “Understanding the CDC’s updated HIV test protocol,” identifies the BioPlex2200 HIV Ag-Ab assay as “the first fifth-generation (HIV) assay.” Clearly, the single most important defining characteristic for each new generation of HIV assays is a reduction in the window period [i.e., the time period between HIV infection and the presence of detectable HIV-RNA or HIV antibodies/antigen (p24)], not the ability to differentiate between HIV-1 and HIV-2 infection in a single assay. Discriminating between HIV-1 and HIV-2 infection is clinically important, despite the fact that the prevalence of HIV-2 infection in the U.S. is low compared to HIV-1 subtype M infection. During the 22-year period, 1987-2009, only 166 cases of HIV-2 infection were reported in the United States by the Centers for Disease Control and Prevention (CDC), while in 2011 alone, the CDC estimated that the number of HIV-infected individuals was 1.2 million.1,2
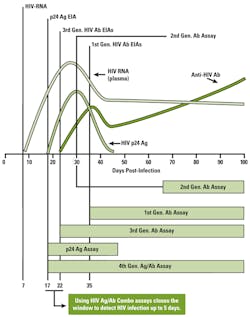
The reduction in the window period of up to five days by fourth-generation HIV assays has been achieved by the development of an HIV “combo” assay that detects both HIV antibodies and the HIV p24 antigen. The serologic profile of p24 antigen in the time-course of HIV infection demonstrates an earlier rise and peak in plasma concentration than occurs with HIV antibodies (Figure 1).
The principal difference between the BioPlex 2000 HIV Ag-Ab assay and currently available fourth-generation HIV assays is the ability to discriminate between HIV-1 and HIV-2 infection in a single assay. Notably, Mr. Kapler’s article provides information on the window period for first-, second-, third-, and fourth-generation HIV assays, yet no information, data, or reference citations are provided indicating the window period for the BioPlex 2000 HIV assay. In my view, and for reasons indicated above, earlier detection of HIV infection, HIV-1 or HIV-2, is a more important characteristic of an HIV screening assay than the ability to differentiate simultaneously whether a positive HIV test result is due to HIV-1 or HIV-2, especially given the markedly low prevalence of HIV-2 infection in the United States.
I suggest that we reserve the definition of a new and improved HIV assay, deserving of the moniker fifth-generation, for an HIV assay that reduces the window period below that achieved with a fourth-generation assay. If that can be achieved with the ability to simultaneously differentiate in a single assay between HIV-1 and HIV-2, then this is an added bonus of such an assay; otherwise, we need to change the currently prevailing definition of a new “generation” of an HIV assay. However, a change in definition based solely (assuming there are no data indicating a reduction in the window period for the BioPlex 2000 HIV assay versus current fourth-generation HIV assays) on an analytical characteristic of any HIV assay (e.g., the type of instrument, the principle of the method, etc.) is not consistent with our current usage of new “generation” of an HIV assay.
–Frank H. Wians, Jr., PhD, AT(ASCP), MASCP,
DABCC, FACB
Professor of Pathology
Paul L. Foster School of Medicine
Texas Tech University Health Sciences Center, and Technical Director, Clinical Chemistry,
University Medical Center
El Paso, TX
REFERENCES
- MMWR 2011;60(29):985-988.
- MMWR 2015;64(24):657-662.
- Wians, FH Jr, Moore HA, Briscoe D, Anderson KM, et al. Evaluation of four qualitative third-generation HIV antibody assays and the fourth-generation Abbott HIV Ag/Ab Combo Test. Lab Med 2011;42(9):523-535.
Author’s response
Dr. Wians makes the case that the BioPlex 2200 HIV Ag-Ab assay does not deserve fifth-generation status because it does not significantly reduce the window period of infectivity detection. I appreciate his close reading, but that argument misses the mark. The assay in question deserves next-generation status because it is the first assay that can simultaneously detect and differentiate the p24 antigen and antibodies to HIV-1 and HIV-2—in one set of results. Therefore, the assay can tell a clinician not only the type of HIV infection but also the patient’s stage of infectivity (acute vs. established). This should significantly reduce what I might call the “treatment window”— the time period between overall HIV detection and the initiation of correct treatment. For the record, the assay’s window period is ~12 days. (Disclosure: I work part time for its maker, Bio-Rad, through an outside consulting firm).
He further suggests that earlier detection of HIV infection of either type “is a more important characteristic of an HIV screening assay” than whether the type of infection is HIV-1 or HIV-2, because of the rarity of HIV-2 in the U.S. He concedes elsewhere, however, that distinguishing between HIV-1 and HIV-2 is “clinically important.” In this regard he is trying to have it both ways. Either it is important or it isn’t, whether the type is revealed during the initial screening phase or the differentiating phase of the algorithm. As he knows, the CDC—like the FDA—takes actions based largely on the precautionary principle, which has been a component of public health policy since the AIDS epidemic began. Two of the CDC’s stated objectives in changing the algorithm were to identify infections earlier and to more accurately diagnose HIV-2 because it had been misdiagnosed under the former testing paradigm. Certainly, there were only 166 cases of HIV-2 in 22 years. That doesn’t mean much statistically. It only matters to the person infected with HIV-2 who is desperately seeking the right medicine.
More generally, his argument sits atop two faulty premises: that some official entity confers generational status on HIV assays, and that such status is based solely on the criterion of the infection-detection window. I have found no evidence that the CDC, or any other agency, body, or authority, confers generational status on new HIV assays. That status, like the criteria used to determine a new generation, filters up from the laboratory/research/manufacturing community itself. And filter it has. None other than Bernard Branson, MD, former Associate Director for Laboratory Diagnostics in the CDC’s Division of HIV/AIDS Prevention, has in at least one public presentation referred to the assay in question as a
fifth-generation assay.
–Robert Kapler