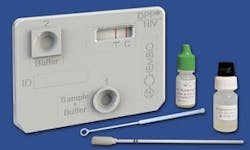
Chembio’s DPP HIV 1/2 Assay
For more information, please visit: http://chembio.com/products/human-diagnostics/dpp-hiv-12-assayx/
Hemosure’s One-Step Immunological Fecal Occult Blood Test
For more information, please visit: http://hemosure.com/hospital-laboratory/products/
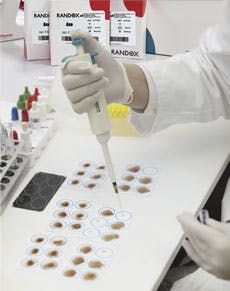
Randox’s range of rapid tests
For more information, please visit: http://www.randox.com/reagents/rapid-tests-serology
Roche’s cobas Influeza A/B Test
For more information, please visit: https://usdiagnostics.roche.com/en/instrument/cobas-liat.html
Thermo Scientific’s UltraVision Quanto Detection System
For more information, please visit: http://www.thermoscientific.com/en/product/ultravision-quanto-detection-system-hrp-dab.htmt
Verax Biomedical’s Platelet PGD Test
For more information, please visit: http://www.veraxbiomedical.com/products/platelet-pgd-test.asp
Hardy Diagnostics PYR Test Kit
For more information, please visit: https://catalog.hardydiagnostics.com/cp_prod/product/z175-pyr-test-kit-100-tests-with-20ml-chromogenic-developer-on-paper-disks-by-hardy-diagnostics-prefer-to-ship-ground-test-disks-strips-reagents
Carolina Liquid Chemistries Corp.
For more information, please visit: http://carolinachemistries.com/CLC/index.cfm/hurl/idsPageID=133/Type=/Carolina-Liquid-Chemistries-Home