Laboratory testing has grown from a manual, “hands-on” process providing a simple test menu to an instrument-centric, high-volume clinical engine inside the modern healthcare enterprise. While the instruments have grown both in size and scale, the proverbial “glue” that holds the laboratory together is the lines of automation. These “conveyor belts” for laboratory samples bring fast and accurate routing of specimens to specific points in the laboratory work flow. Because of this growth, automation has moved past the “nice to have” to the “must have” in the modern laboratory, and automation solutions are beginning to extend their footprints into other areas of the laboratory such as microbiology and molecular diagnostics. This article will cover the genesis of automation in laboratory medicine, narrate the evolution of the designs and principles of automation, and highlight the future directions for the field.
Brief history of lab automation
Laboratory automation began in the 1950s, and its history includes the evolution of laboratory instruments, the growth of the laboratory information (management) systems (LIS/LIMS), and development of pre- and post-analytic automation.
The first automated instrument was introduced in 1957. This instrument, the Autoanalyzer I, utilized continuous flow analysis (CFA) to dramatically increase the instrument throughput (20 samples at a time) to leap past the manual processes of the time. This one instrument started the drive to automate the clinical laboratory work flow at the level of the assay. Following the Autoanalyzer I, instruments like the SMA (Sequential Multiple Analyzer, 1969) and SMAC (Sequential Multiple Analyzer with Computer, 1974) came to market with larger test menus and higher throughputs.1 This trend continues today with each new generation of instruments that elevate the volume of work and menu of assays.
The laboratory information systems/laboratory information management systems began to take shape in the 1970s. LIS/LIMS platforms brought electronic data management to the laboratory to manage the work flow and electronic interfaces to the instruments. Driven by an initial need to accurately capture billing codes, these systems helped automate laboratory operation by removing paper-based logs and capturing data electronically, directly from laboratory instruments. Coupled with automated instruments, the throughput of the clinical laboratory dramatically increased around the instruments. This set the stage for extensions of automation beyond the instruments because labs now had instruments that needed specimens at a rate greater than what a laboratorian could provide.
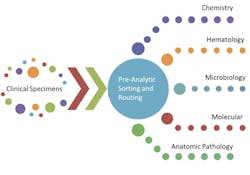
After the start of instrument automation and electronic automation via the LIS/LIMS, attention fell on the pre- and post-analytic portions of the laboratory work flow. These parts of the work flow comprise all of the necessary steps to accept a clinical specimen into the laboratory for testing, the processing steps to prepare the sample for testing, and the steps for final disposition of the specimen. The typical pre-analytic work flow involves some form of specimen identification (perhaps accessioning and labeling if not already done) followed by routing to the correct part of the laboratory. The sorting process is depicted in Figure 1.
Figure 1. Pre-analytic sorting process
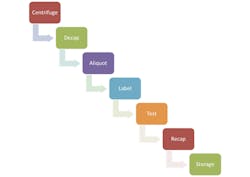
As the specimen nears the instrument for testing, other processing steps like centrifugation, de-capping, and aliquoting can happen in an automated fashion to prepare the sample for testing. After testing, the specimen can be re-capped and sent off for long-term storage. These typical steps in those work flows surrounding the test are shown in Figure 2.
Figure 2. Typical steps in work flow
Despite having automated ordering, resulting, and test performance, the pre- and post-analytic work flows were highly manual processes at the end of the 1970s. Laboratory staff performed these necessary steps to move the specimens through the laboratory. The pre-analytic phase alone is estimated to have consumed 60% of the time and effort in the total specimen work flow. The estimates of the contribution of pre-analytic error to total laboratory error range from 30% to 86%.2-4
Given the large impact a pre-analytic error can have on testing, efforts to automate the pre-analytic phase began soon after the automated instrument and LIS/LIMS systems entered the laboratory. Masahide Sasaki, MD, PhD, is credited as being the “father of laboratory automation.” His work in the 1980s with conveyor belts, circuit boards, and programmable robots created the first automated laboratory.5 This opened the door for the expansion of automation outside the confines of the instrument to reach the pre-analytic work flow and the post-analytic work flow.
Present state of laboratory automation
With the three components of automation growing within the clinical laboratory in the late 1980s (instrument-level automation, the LIS/LIMS, and early pre- and post-analytic automation), the strategies for laboratory automation have taken a variety of routes. First, the degree of automation at the level of the instrument has grown to handle higher throughputs as the demands of the laboratory have grown. This growth is easily seen in the orders of magnitude increases in instrument throughput over the past few decades. The LIS/LIMS systems have grown from simple appliances that handle the mechanics of laboratory billing to complete, end-to-end platforms that govern the entire business process and work flow of the laboratory. These IT systems are now mission-critical to the operation of a laboratory and must work in concert with instruments and automation for a laboratory to be successful.
The pre- and post-analytic automation solutions are present in one of two varieties: open and closed. Of the two, closed automation is the more common. Closed automation solutions are those that are provided by instrument manufacturers and typically connect only to instruments from that vendor. And unless a single vendor has instruments in multiple parts of the lab, closed automation solutions exist as islands of automation around their specific parts of the lab. Open automation solutions exist independently from the instruments in the laboratory. These solutions are built by independent companies and interface to the instruments and the LIS/LIMS to automate the pre- and post-analytic work flows.
The main difference between the open and closed approaches is the types of instruments connected to the automation line. Unlike the closed lines that are provided with instruments, the open automation solutions are designed and acquired by labs independently of the instruments in the laboratory. While the open automation lines connect to instruments in a similar fashion (direct track sampling or robotic arm), open automation lines can cross service areas of the lab because of the ability to interface to any instrument, regardless of the vendor. This independence from a specific vendor allows open automation systems to transport specimens across various parts of the lab and provide a single automation solution for the entire laboratory.
The current “whys” for automation in the laboratory are error reduction and staff augmentation. Both are driven by increased testing demands and laboratory staffing needs. Automation has indeed moved from “nice to have” for large reference laboratories to “must have” for any clinical laboratory. The current climate for reimbursements for laboratory testing has only increased the importance of automation, with further reductions that push laboratories to optimize laboratory throughput and staffing to keep up with the increasing demands.
Future directions
Every component of laboratory work flow in all disciplines is at some stage of automation. While the fields of clinical chemistry and hematology were the first to be fully automated, the realms of molecular diagnostics and anatomic pathology will soon follow. You can see the initial starts in each field with automated instruments such as immunostainer platforms in anatomic pathology or DNA extractors in molecular diagnostics. Microbiology is just now beginning the journey with the arrival of fully automated microbiology platforms that automate the entire work flow with one system. With the convergence of the disciplines of laboratory medicine (anatomic pathology, clinical pathology, molecular diagnostics) that is driving the movement of specimens across disciplines, laboratory automation will need both to expand and to connect all parts of the laboratory as well.
References
- Streitberg GS, Bwititi PT, Angel L, Sikaris KA. Automation and expert systems in a core clinical chemistry. JALA. 2009:14(2):94-105.
- Wiwanitkit V. “Types and frequency of preanalytical mistakes in the first Thai ISO 9002: 1994 certified clinical laboratory, a 6-month monitoring.” BMC Cli. Patho., 2001;1:5. doi:10.1186/1472-6890-1-5.
- Guder W. Preanalytical factors and their influence on analytical quality specifications. Scand J Clin Lab In. 1999;59(7):545-549.
- Plebani, M. “Errors in clinical laboratories or errors in laboratory medicine?” Clin Chem Lab Med. 2006;44(6):750-759.
- “Masahide Sasaki, MD, PhD. August 27, 1933–September 23, 2005.” Clin Chem April 2006; 52(4):791-792.