The clinical application of islet autoantibody testing for the diagnosis of autoimmune diabetes
Type 1 diabetes is highly prevalent in the general population affecting, at a minimum, approximately 1 in 300 children. Because islet autoimmunity can be detected in ~10% of patients initially presenting with diabetes that is not insulin dependent (e.g., type 2 diabetes), the overall frequency of autoimmune diabetes may be as high as 1% to 2% of the adult population.1 Because the diagnosis of autoimmune diabetes is not always straightforward, laboratorians should understand the biology, detection, and clinical uses of islet autoantibody determinations.2 We present the basics in a question-and-answer format.
What is type 1 diabetes? Diabetes mellitus is a descriptive term for a family of disorders that are characterized by chronic hyperglycemia and the development of long-term complications.3 These complications affect the large blood vessels (e.g., accelerated atherosclerosis), small vessels (e.g., nephropathy and retinopathy) and the nervous system (e.g., neuropathies). Diabetes during pregnancy poses health problems for the mother and the fetus.
Diabetes is classified as type 1, type 2, gestational diabetes, and other specific types of diabetes.4 Overall there are more than 40 different types of diabetes. Excluding gestational diabetes, the most common forms of diabetes are type 1 diabetes (~10% of all cases of diabetes) and type 2 diabetes (~90% of all cases of diabetes). Other specific types of diabetes may account for ~3% or more of all cases of diabetes.5
Type 1 diabetes results from an absolute deficiency of insulin (a.k.a., insulinopenia) caused by autoimmune destruction of the pancreatic beta cells. Whereas autoantibodies are important markers of islet autoimmunity, the actual destruction of the beta cells results from a cell-mediated autoimmune response.
How is diabetes diagnosed? Excluding pregnancy, the biochemical criteria for the diagnosis of diabetes are the same irrespective of the type of diabetes.6 Patients who present in diabetic ketoacidosis (DKA) or hyperglycemic-hyperosmolar state (HHS), or patients who have a random plasma glucose of 200 mg/dL or more and frank symptoms of hyperglycemia (e.g., polyuria, polydipsia, polyphagia, weight loss, poor wound healing, blurry vision, etc.) are diagnosed with diabetes. In the absence of these criteria, diabetes is diagnosed when hyperglycemia or an elevated hemoglobin A1c level are detected on at least two separate occasions. Hyperglycemia is defined as a fasting plasma glucose of 126 mg/dL or more, or a two-hour plasma glucose of 200 mg/dL or more following a 75 g glucose load (e.g., during an oral glucose tolerance test [OGTT]). During an OGTT children should receive 1.75 g/kg of glucose to a maximum of 75 g. A hemoglobin A1c level is elevated at 6.5% or greater. Note that according to the American Diabetes Association (ADA), neither insulin nor C-peptide is used for the diagnosis or classification of diabetes.
In the majority of cases of type 1 diabetes in children, the severity of hyperglycemia is profound and the diagnosis of diabetes is usually little in doubt. About 30% of children diagnosed with type 1 diabetes present acutely in DKA. Although not usually required for the diagnosis of diabetes, hemoglobin A1c is often measured at the time of diagnosis of diabetes and is usually quite elevated. The distinction between type 1 diabetes and type 2 diabetes is made clinically by the treating physician.
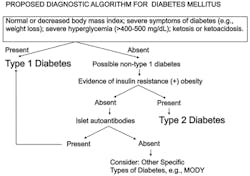
What factors favor the diagnosis of type 1 diabetes? Factors that favor the diagnosis of type 1 diabetes include youth-onset; weight loss at presentation; leanness (e.g., a normal or low body mass index); severe hyperglycemia; ketonuria or DKA; and lack of insulin resistance. Family history is not helpful in favoring the diagnosis of type 1 diabetes over type 2 diabetes because in only 15% or less of cases of type 1 diabetes is there a family history of type 1 diabetes.
What factors favor the diagnosis of type 2 diabetes? Factors that favor the diagnosis of type 2 diabetes include onset in adulthood; obesity; less severe hyperglycemia; absence of ketonuria or DKA; evidence of insulin resistance; and usually a strong family history of type 2 diabetes.
Do the above factors definitively distinguish type 1 diabetes from type 2 diabetes? Despite these characteristics, many times patients “do not read the textbooks” and cannot be neatly (and easily) partitioned into type 1 diabetes or type 2 diabetes. For example, because the general population frequency of obesity is very high, patients with type 1 diabetes may be obese at presentation. Because of obesity, there is a rising frequency of type 2 diabetes in children and adolescents. Therefore age at onset of diabetes is less and less important in distinguishing type 1 diabetes from type 2 diabetes. Twenty-five percent of cases of type 1 diabetes do occur after age 18. Adolescent minority youth who do not recognize the symptoms of diabetes and do not seek medical care at an early stage of the disease may ultimately present at an advanced stage of metabolic decomposition including DKA that can be fatal.7,8
Do insulin or C-peptide levels distinguish type 1 diabetes and type 2 diabetes? Studies comparing insulin and C-peptide levels in type 1 diabetes and type 2 diabetes patients show extensive overlap and are generally difficult to interpret. Certainly, if the insulin or C-peptide levels are very low, the diagnosis of type 1 diabetes is favored. As well, if the insulin or C-peptide levels are very high, the diagnosis of type 2 diabetes is favored. Nevertheless, for a large proportion of people with diabetes, insulin and C-peptide levels are not reliable in differentiating these disorders.9-12
If clinical and metabolic criteria do not always distinguish type 1 diabetes and type 2 diabetes, how then can type 1 diabetes and type 2 diabetes be separated? If the physician is in doubt about the type of diabetes that the patient has, islet autoantibodies are detected in 95% or more of cases of new-onset type 1 diabetes.13 Because no single islet autoantibody is present in more than 80% of new-onset patients with type 1 diabetes, clinicians find it useful to order a panel of islet autoantibody tests when the diagnosis of autoimmune diabetes is sought.14 Patients lacking autoimmune diabetes are negative for islet autoantibodies. Figure 1 provides a suggested approach to the diagnosis of specific forms of diabetes.
Why is it important to diagnose type 1 diabetes versus other types of diabetes? Treatment depends upon the specific diagnosis. Type 1 diabetes is treated with exogenous insulin replacement.15 Type 2 diabetes is treated through weight loss, exercise, and diet. If such non-pharmacologic treatments do not lower the plasma glucose level and hemoglobin A1c sufficiently, there is a growing list of anti-diabetic medications that are available to treat type 2 diabetes. While the sulfonylureas are the oldest drugs used (excluding insulin), metformin is the drug recommended to initially treat type 2 diabetes.16 Metformin increases peripheral insulin sensitivity and reduces hepatic glucose output.
It is important to diagnose type 1 diabetes because other autoimmune diseases can occur in high frequency in people with type 1 diabetes.17 These associated disorders include autoimmune thyroid disease (AITD), chronic lymphocytic gastritis producing pernicious anemia, and celiac disease. The most common manifestation of AITD is Hashimoto thyroiditis. Graves disease is a less common form of AITD. A less common association than AITD, pernicious anemia, or celiac disease is autoimmune Addison disease. Despite the fact that Addison disease is the least common of the four associated diseases listed, unrecognized Addison disease that progresses to Addisonian crisis can be fatal.
The autoimmune disorders associated with type 1 diabetes can be predicted by the presence of their associated autoantibodies. Therefore many physicians will routinely screen their patients with type 1 diabetes for AITD by testing for thyroperoxidase (TPOA) and thyroglobulin (TGA) autoantibodies, and will screen for chronic lymphocytic gastritis by testing for gastric parietal cell autoantibodies or intrinsic factor autoantibodies.
The need for routine screening for celiac disease is controversial, as type 1 diabetes patients with serologic evidence of celiac disease (e.g., IgA tissue transglutaminase autoantibodies or deaminated gliadin autoantbodies) are usually asymptomatic.18 If a type 1 diabetes patient has either AITD or chronic lymphocytic gastritis, adrenal autoimmunity should be sought by screening for adrenal cell cytoplasmic autoantibodies or autoantibodies to the enzyme 21-hydroxylase. If adrenal autoantibodies are detected, the presence of an autoimmune polyglandular syndrome should be entertained.19
If AITD is suggested by the presence of TPOA or TGA, yearly measurements of thyroid-stimulating hormone (TSH) should be obtained. If gastric parietal cell autoantibodies or intrinsic factor autoantibodies are identified, yearly measurements of ferritin and vitamin B12 are indicated. In search of nascent Addison disease, cortisol following cosyntropin injection and renin should be measured on a yearly basis.
The diagnosis of type 1 diabetes also has implications for family members. In the research setting, family members can be screened for serological evidence of islet autoimmunity that may allow them to enter into prevention trials such as conducted by the NIH-supported Type 1 Diabetes TrialNet study. If AITD or chronic lymphocytic gastritis is detected in the type 1 diabetes proband, the first-degree family members should be screened for such conditions, because AITD and chronic lymphocytic gastritis are strongly familial.
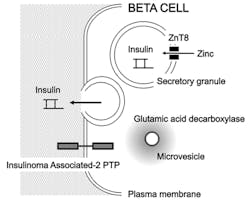
What are islet autoantibodies? An islet autoantibody is an autoantibody that targets autoantigens that are on the surface of islet cells or within islet cells, or are products of islet cells (Table 1). Only one islet autoantigen, insulin, is absolutely beta-cell specific. Islet cell surface autoantibodies (ICSA) are difficult to detect, and this technology is not used in clinical practice. Several cytoplasmic autoantigens exist and will be subsequently discussed. As well as being beta-cell specific, insulin is a secreted beta-cell autoantigen.
Table 1. Autoantigens detected by islet autoantibodies
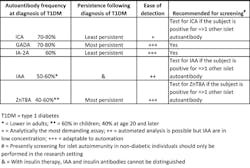
There are now five islet autoantibodies that can be readily detected in type 1 diabetes (Figure 2). There are commercial kits available for all of these determinations, or the assays can be run in reference laboratories. Some laboratories have developed these assays as in-house methods. These five islet autoantibodies are islet cell cytoplasmic autoantibodies (ICA); insulin autoantibodies (IAA); glutamic acid decarboxylase autoantibodies (GADA); insulinoma associated-2 autoantibodies (IA-2A); and zinc transporter-8 autoantibodies (ZnT8A).
What are the characteristics of ICA? ICA were initially described in 1974 as being present in patients with autoimmune polyendocrine deficiencies.20 A few years later, ICA were described in patients with new-onset type 1 diabetes. With this history in mind, ICA were the first islet autoantibodies to be described.
ICA remain a gold standard to which other islet autoantibodies are often compared. ICA are sensitive and specific for type 1 diabetes. Whereas the other islet autoantibodies may be found in the general population in frequencies of up to 1% to 3%, ICA are rare in the general population. In a study of approximately 10,000 schoolchildren in Pasco County, Florida, our research group found that the general population frequency of ICA in children was only ~1 in 250 (0.4%).21
ICA are present in 70% to 80% of individuals with new-onset type 1 diabetes. Following the diagnosis of type 1 diabetes, ICA frequency declines to less than 5% to 10% by 10 years. Similar to other islet autoantibodies, ICA are very commonly present prior to the diagnosis of type 1 diabetes. In this way, ICA in non-diabetic individuals predicts a markedly increased risk of type 1 diabetes. In the Pasco County school study, at least 50% of ICA positive children developed type 1 diabetes during follow-up.
If ICA are detected in subjects who were previously diagnosed with type 2 diabetes, such diabetic subjects really have a slowly progressive form of autoimmune diabetes that has been described as “latent autoimmune diabetes of adulthood (LADA).”22,23 Indeed, ICA can be detected in up to 10% to 15% of people with initially diagnosed type 2 diabetes.
LADA is a type of autoimmune diabetes where the rate of loss of insulin secretion is not abrupt (as in cases of type 1 diabetes with onset observed in children and adolescents), and the initial presentation is that of only mild hyperglycemia, usually without ketosis. Subjects with LADA often require exogenous insulin administration for control of their diabetes after failing to respond to oral anti-diabetic drugs. From a genetic viewpoint, LADA subjects are similar to individuals with type 1 diabetes displaying increased frequencies of HLA-DR3 and HLA-DR4. Besides ICA, other islet autoantibodies can be detected in LADA subjects such as GADA and ZnT8A.
ICA are detected by indirect immunofluorescence using human, blood group O pancreas from donors less than 40 to 45 years of age. The donors must not have a history of diabetes themselves and should have had normal renal function immediately prior to their demise. A history of illicit drug use should also exclude subjects from serving as donors.
The ICA procedure is labor-intensive and technically challenging. For ICA testing, human pancreas is preferred over the use of non-human primate pancreas.24 Because of the analytical demands of ICA testing, ICA testing should only be undertaken by laboratories that, first, perform large numbers of ICA tests, and, second, pursue rigorous internal and external quality assurance and quality control programs. ICA results are reported in JDF (Juvenile Diabetes Foundation) units.
What are the characteristics of IAA? Autoantibodies to insulin (IAA) were first described in 1983.25 Such autoantibodies are usually measured using immunoprecipitation of patient sera after addition of radioactive insulin. The counts in the precipitant are then determined. More counts correspond to higher IAA levels. The IAA assay does not distinguish IAA from antibodies induced from insulin administration. Therefore IAA should preferably be assayed prior to instituting exogenous insulin therapy and should not be sought if insulin has been administered for more than one week.
Of the five islet autoantibodies discussed in this article, IAA are the least common autoantibodies at disease onset.26 Their frequency in new-onset type 1 diabetes children is 50% to 60%, and in adults, IAA are uncommon at disease onset. As compared to other islet autoantibodies, IAA are the least disease-specific markers, as IAA can be detected in some individuals with autoimmune thyroid disease, chronic hepatitis, and other autoimmune diseases. Annual or biannual workshops [e.g., the Islet Autoantibody Standardization Program (IASP)] for islet autoantibody testing are available for IAA, GADA, IA-2A, and ZnT8A.
What are the characteristics of GADA? Following ICA and IAA, autoantibodies to glutamic acid decarboxylase (GAD) were next discovered. There are two forms of GAD encoded by separate genes: a 65 kDa form (GAD65) and a 67 kDa form (GAD67). GADA are most often directed against the 65 kDa form. For this reason, GADA are sometimes referred to as “GAD65 autoantibodies.”
The initial description of GADA is quite interesting.27 It was known that about one-third of people with a neurologic disorder known today as “stiff person syndrome” had type 1 diabetes. Stiff person syndrome, which can be disabling, is characterized by variable rigidity of the muscles in the limbs and trunk that can be precipitated by stress and environmental triggers such as loud noises. When GADA were detected in stiff person patients, and because stiff person syndrome is strongly associated with type 1 diabetes (as well as other autoimmune diseases), GADA were sought, and found, in people with type 1 diabetes who were not affected by stiff person syndrome.
Like ICA, GADA are found in 70% to 80% of individuals with new-onset type 1 diabetes. As well, GADA in non-diabetic individuals predicts the later development of type 1 diabetes. GADA are not as specific as ICA for the diagnosis of autoimmune diabetes because the general population frequency of positivity for GADA is up to 3%. For the detection of LADA, GADA are actually more sensitive than ICA. This is because GADA are more persistent following the diagnosis of autoimmune diabetes. The next most common autoantibody in LADA is ZnT8A.
GADA are often detected by radioimmunoprecipitation assays, although nonradioactive formats are available. ELISA kits for GADA space the GAD protein out from the wall of the well. Otherwise, if GAD is attached directly to the wall of the well, GADA are not generally detected, as the GAD may be denatured or modified in a way that its auto-epitopes are not available for binding by the autoantibodies.
What are the characteristics of IA-2A? Insulinoma associated-2 autoantibodies (IA-2A) were initially discovered when researchers were screening proteins produced by insulinoma cells for their reactivity with sera from subjects with type 1 diabetes. The IA-2 autoantigen is a protein tyrosine phosphatase transmembrane, cell surface molecule that may be involved in neuroendocrine secretory processes.28 The first IA-2 autoantibodies were described against its intracellular domain, leading to their description as “ICA512 autoantibodies.” Therefore in the various immunoprecipitation immunoassays, when the full-length autoantigen is used, labs commonly describe these autoantibodies as “IA-2A,” whereas if only the intracellular domain is used in the assay, the autoantibodies can be described as ICA512 autoantibodies.
At onset of type 1 diabetes, IA-2A are not as common as ICA or GADA, being present in ~60% of subjects; however, IA-2A are more common, in general, than IAA. Testing for IAA, GADA, and IA-2A can be highly automated (versus ICA testing, which is strictly manual in nature) and thus any discussion of population screening begins with these three autoantibodies.
What are the characteristics of ZnT8A? The newest islet autoantibodies entering the realm of routine clinical usefulness are directed against transporter-8 that moves zinc from the cytoplasm to the insulin-containing secretory granules.29 Therefore these autoantibodies are termed zinc transporter-8 autoantibodies (ZnT8A). The “official” name for this transporter is “solute carrier family 30 (zinc transporter), member 8” (SLC30A8).
Between ages 4 and 20, ZnT8A are present in ~60% to 70% of new-onset type 1 diabetes cases, whereas after age 20, the frequency of ZnT8A positivity declines to ~40% in new-onset subjects. ZnT8A measurements can be helpful when other islet autoantibodies are negative in suspected new-onset type 1 diabetes cases: ZnT8A are present in 14% of new-onset patients negative for GADA, IA-2A, and IAA. ZnT8A are useful in the diagnosis of LADA as ZnT8A are nearly as common as GADA in LADA patients.30 Another novel use of ZnT8A testing is that ZnT8A positivity in type 1 diabetes patients who have received a pancreas transplant predicts graft failure.31
An interesting aspect of ZnT8A is that genetic variations of the zinc transporter-8 exist.32 Therefore it is advised that the immunoassays for ZnT8A include these variants as autoantigens. The commercially available kit for ZnT8A detection uses a non-radioactive ELISA format.
What are the indications for measuring islet autoantibodies? As discussed above, when the clinician cannot clearly differentiate type 1 diabetes from type 2 diabetes based upon the clinical features identified in the patient (e.g., severity of hyperglycemia, presence of ketosis, obesity, etc.), islet autoantibody testing is warranted because the correct diagnosis affects the selection of therapy, the patient’s prognosis, and the need for testing for associated autoimmunities (both endocrine and non-endocrine). Furthermore, for family members, their risk of type 1 diabetes and related autoimmunities rises when the proband is diagnosed with type 1 diabetes.
Should single islet autoantibody tests be ordered, or should panels be ordered? Because no single islet autoantibody is present in more than 70% to 80% of new-onset subjects (e.g., ICA and GADA), it is advised that panels of autoantibodies be ordered. If a single islet autoantibody or multiple islet autoantibodies are detected, the diagnosis of an autoimmune form of diabetes is confirmed. There is no way to predict which autoantibody or autoantibodies an individual might have other than to state that IAA are uncommon in adults. Therefore laboratories can construct two suggested panels. The “pediatric islet autoantibody panel” would test for ICA, IAA, GADA, IA-2A, and ZnT8A, whereas the “adult islet autoantibody panel” would test for ICA, GADA, IA-2A, and ZnT8A. When LADA is of concern, GADA and ZnT8A testing would be appropriate. The characteristics and uses of islet autoantibodies are summarized in Table 2.
What is the future for islet autoantibody testing? Researchers are working diligently to develop methods to prevent type 1 diabetes. This is the goal of the Type 1 Diabetes TrialNet study. Once type 1 diabetes can be prevented, the case can be made that all children should be screened for islet autoantibodies on multiple occasions to identify those children at highest risk for developing type 1 diabetes.33 These children would then be treated to prevent type 1 diabetes. Such treatments may involve altering environmental factors that could precipitate type 1 diabetes, suppressing the destructive autoimmune process that otherwise kills beta cells, or inducing immunologic tolerance to beta-cell autoantigens.
References
- Winter W, Chihara T, Schatz DA. The genetics of autoimmune diabetes. Am J Dis Child. 1993;147:1282-1290.
- Winter WE, Schatz DA. Autoantibody markers in diabetes. Clin Chem. 2011; 57:168-175.
- Winter WE, Hardt NS, Harris NS. Carbohydrate disorders. In: WA Clarke, ed. Contemporary Practice in Clinical Chemistry. 2nd ed. Washington, DC: AACC Press. 2011;343-357.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabet Care. 2013;36:S67-S74.
- Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue K; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2006-2007. The diagnosis and management of monogenic diabetes in children. Pediatr Diabet. 2006;7(6):352-360.
- Winter WE, Harris NS. Evaluation of hyperglycemia. In: WE Winter, L Sokoll I Jialal, eds. Handbook of Diagnostic Endocrinology. 2nd ed. Washington, DC: AACC Press. 2008;265-294.
- Morales AE, Rosenbloom AL. Death caused by hyperglycemic hyperosmolar state at the onset of type 2 diabetes. J Pediatr. 2004;144(2):270-273.
- Zdravkovic V, Daneman D, Hamilton J. Presentation and course of type 2 diabetes in youth in a large multi-ethnic city. Diabet Med. 2004;21(10):1144-1148.
- Berger B, Stenström G, Sundkvist G. Random C-peptide in the classification of diabetes. Scand J Clin Lab Invest. 2000;60(8):687-693.
- Hosszúfalusi N, Vatay A, Rajczy K, et al. Similar genetic features and different islet cell autoantibody pattern of latent autoimmune diabetes in adults (LADA) compared with adult-onset type 1 diabetes with rapid progression. Diabet Care. 2003;26(2):452-457.
- Bolinder J, Fernlund P, Borg H, et al. Hyperproinsulinemia segregates young adult patients with newly diagnosed autoimmune (type 1) and non-autoimmune (type 2) diabetes. Scand J Clin Lab Invest. 2005;65(7):585-594.
- Katz LE, Jawad AF, Ganesh J, Abraham M, Murphy K, Lipman TH. Fasting C-peptide and insulin-like growth factor-binding protein-1 levels help to distinguish childhood type 1 and type 2 diabetes at diagnosis. Pediatr Diabet. 2007;8(2):53-59.
- Simone E, Eisenbarth GS. Chronic autoimmunity of type I diabetes. Horm Metab Res. 1996;28(7):332-336.
- Zhang L, Eisenbarth GS. Prediction and prevention of type 1 diabetes mellitus. J Diabet. 2011;3(1):48-57.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabet Care. 2013;36:S11-S66.
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabet Care. 2006;29(8):1963-1972.
- Maclaren NK, Riley WJ. Thyroid, gastric, and adrenal autoimmunities associated with insulin-dependent diabetes mellitus. Diabet Care.1985;8 (suppl 1):34-38.
- Sud S, Marcon M, Assor E, Palmert MR, Daneman D, Mahmud FH. Celiac disease and pediatric type 1 diabetes: diagnostic and treatment dilemmas. Int J Pediatr Endocrinol. 2010. doi10.1155/2010/161285
- Haller MJ, Winter WE, Schatz DA. Autoimmune polyglandular syndromes, In: Mark Sperling, ed. Pediatric Endocrinology. 3rd ed. Philadelphia, PA: W.B. Saunders. 2008;770-787.
- Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2(7892):1279-1283.
- Maclaren NK, Lan MS, Schatz D, Malone J, Notkins AL, Krischer J. Multiple autoantibodies as predictors of type 1 diabetes in a general population. Diabetologia. 2003;46(6):873-874.
- Zimmet PZ, Tuomi T, Mackay IR, et al. Latent autoimmune diabetes mellitus in adults (LADA): the role of antibodies to glutamic acid decarboxylase in diagnosis and prediction of insulin dependency. Diabet Med.1994;11(3):299-303.
- Turner R, Stratton I, Horton V, et al; UK Prospective Diabetes Study Group. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet. 1997;350(9087):1288-1293.
- Scherbaum WA, Trischler G, Pfeiffer EF. Non-human primate pancreas as a substrate for the detection of islet-cell antibodies in human sera. Diabetes Res Clin Pract. 1989;20;7(1):1-5.
- Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222(4630):1337-1339.
- Atkinson MA, Maclaren NK, Riley WJ, Winter WE, Fisk DD, Spillar RP. Are insulin autoantibodies markers for insulin-dependent diabetes mellitus? Diabetes. 1986;35(8):894-898.
- Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151-156.
- Lan MS, Wasserfall C, Maclaren NK, Notkins AL. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1996;93(13):6367-6370.
- Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104(43):17040-17045.
- Trabucchi A, Faccinetti NI, Guerra LL, et al. Detection and characterization of ZnT8 autoantibodies could help to screen latent autoimmune diabetes in adult-onset patients with type 2 phenotype. Autoimmunity. 2012;45(2):137-142.
- Occhipinti M, Lampasona V, Vistoli F, et al. Zinc transporter 8 autoantibodies increase the predictive value of islet autoantibodies for function loss of technically successful solitary pancreas transplant. Transplantation. 2011;92(6):674-677.
- Wenzlau JM, Liu Y, Yu L, et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 2008;57(10):2693-2697.
- Xu P, Beam CA, Cuthbertson D, Sosenko JM, Skyler JS, Krischer JP; DPT-1 Study Group. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high risk population: receiver operating characteristic analysis. Diabet Care. 2012;35(10):1975-1980.
- Nayak RC, Omar MA, Rabizadeh A, Srikanta S, Eisenbarth GS. “Cytoplasmic” islet cell antibodies. Evidence that the target antigen is a sialoglycoconjugate. Diabetes. 1985;34(6):617-619.