Blood stream infections (BSI) are a leading cause of morbidity and mortality in the United States, and they impact both length of hospital stay and associated healthcare costs. Positive blood cultures detected in the laboratory are considered critical events and play a sentinel role in the diagnosis and management of BSI.
Despite improvements to automated laboratory blood culture systems and increased clinical recognition of the presentation of sepsis, BSIs continue to present challenges for clinicians and laboratories alike. The following review is a contemporary update on current epidemiology of sepsis and highlights key laboratory variables associated with laboratory blood cultures.
In the United States, sepsis and sepsis-related events rank among the ten leading causes of death and are the sixth most common reason for hospitalization. Each year, an estimated 750,000 patients develop bacteremia/fungemia.1 In 2011 the Agency for Healthcare Research and Quality identified hospital mortality rates for septicemia at levels eight times higher than mortality derived from other hospital stays, and in 2009 septicemia was deemed the most expensive reason for hospitalization.2 Within hospital intensive care units (ICU) sepsis and sepsis-related events contribute to 40% of total ICU costs.3
Laboratory blood culture systems remain the gold standard test for the identification of microorganisms implicated in BSI. Over the decades significant advancements have been made in laboratory blood culture systems, including the addition of enriched growth media, advances in automated agitation systems, and development of software which allows faster detection of bacterial growth via improved algorithms. Despite these advancements, obtaining blood cultures before initiating anti-infective therapy, and ensuring appropriate fill volumes of 20 to 40 mL of blood per venipuncture, remain key factors in the successful detection of adult bacteremia.4-7
The majority of adult bacteremias are frequently characterized by a low density of microorganisms (often ≤1 CFU/ml). Therefore, adequate blood volume remains among the most important variables in the recommendation of blood culture collection for adults.7 The importance of obtaining appropriate blood volume collection is now highlighted as a metric within CAP recommendations (MIC 22640), which state that the level of blood culture fill volumes should be periodically monitored by the laboratory. To accomplish this, laboratories utilize many methods such as weighing of bottles, manual measurements, and an automated blood volume monitoring software module.
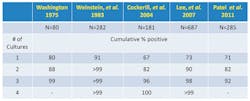
The number of blood cultures required to diagnose BSI is variable and has been debated over the decades. However, evidence suggests that two to three sets of blood cultures (comprised of either an aerobic and anaerobic bottle or two aerobic bottles) should be drawn per septic episode (Table 1). The relevancy of an anaerobic bottle has been proposed by some authors. In 2007, Lassmann et al reported that the mean incidence of anaerobic bacteremia at the Mayo Clinic increased overall by 74% from 1993 to 2004. The authors concluded that anaerobic bacteremia had re-emerged as a significant clinical problem, most likely attributable to the increase in patients with complex underlying diseases such as malignancies and associated chemotherapeutic treatment regimens.8 Patel et al documented that the use of an aerobic/anaerobic paired set of bottles resulted in significantly more pathogens recovered as compared to two aerobic bottles.9
Table 1. Number of blood cultures needed to detect bacteremia and fungicemia
Initiation of prompt, appropriate antimicrobial therapy in patients at risk for sepsis is a clinical goal.10 However, balancing these clinical requirements for prompt antimicrobial therapy with the realities of laboratory cultivation of microorganisms is a contemporary challenge for the clinical laboratory. Initiation of antimicrobial therapy before culture collection may delay pathogen recovery, or in some cases, may artificially sterilize blood cultures. In an effort to address this, blood culture manufacturers have incorporated blood-broth ratios and/or proprietary antimicrobial removal systems into BC media to minimize the impact of antimicrobials present in the media. Some media utilize proprietary resin beads, while others use a blend of Fuller’s earth and activated charcoal, to neutralize the effects of antimicrobials present in blood cultures submitted for laboratory testing.
The implications of improved recovery using these media have been studied.11,12 It is important to recognize the patient population and attributes of systems to understand impacts to recovery rates. Future refinements to existing blood culture media will continue to challenge the impact that contemporary antimicrobial prescribing practices have on the laboratory diagnosis of BSI. Other variables such as collection methods, contamination rates, and rapid, accurate reporting of positive blood cultures can also influence the utility of blood culture testing. By adhering to best practices and actively monitoring quality assurance measures, labs can feel confident that they are providing the best results in supporting clinicians in the diagnosis of sepsis.
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310.
- Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. Hospitals, 2009. Healthcare cost and Utilization Project Statistical Brief #122. October 2011. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf. Accessed May 9, 2013.
- Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112(1):235-243.
- Patel R, Vetter EA, Harmsen WS, Schleck CD, Fadel HJ, Cockerill FR III. Optimized pathogen detection with 30-compared to 20-milliliter blood culture draws. J Clin Microbiol. 2011;49(12):4047-4051.
- Bleck T, Carroll K, Kalil AC, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330-1349.
- Weinstein MP, Doern GV. A critical appraisal of the role of the clinical microbiology laboratory in the diagnosis of bloodstream infections. J Clin Microbiol. 2011;49(9):S26-S29.
- Clinical and Laboratory Standards Institute. Principles and Procedures for Blood Cultures; Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. CLSI document M47-A
- Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
- Patel R, Vetter EA, Harmsen WS, Schleck CD, Fadel HJ, Cockerill FR 3rd. Optimized pathogen detection with 30-compared to 20-milliliter blood culture draws. J Clin Microbiol. 2011;49(12):4047-4051.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
- Flayhart D, Borek AP, Wakefield T, Dick J, Carroll KC. Comparison of BACTEC PLUS blood culture media to BacT/Alert FA blood culture media for detection of bacterial pathogens in samples containing therapeutic levels of antibiotics. J Clin Microbiol. 2007;45(3):816-821
- Zadroga R, Williams DN, Gottschall R, et al. Comparison of 2 blood culture media shows significant differences in bacterial recovery for patients on antimicrobial therapy. Clin Infect Dis. 2013;56(6):790-797.