Molecular
diagnostic (dx) testing has changed the practice of medicine and has
introduced a new type of laboratory to the industry. Like traditional
laboratories, molecular diagnostic laboratories perform a range of
tests, employ skilled laboratorians, issue test reports, and provide
patient information for use by clinicians. In almost every other aspect,
molecular diagnostic laboratories differ from traditional labs and,
based on the types of molecular testing performed, may also differ from
one another. This article describes the growing importance of molecular
testing, provides examples of tests used to guide the treatment of
patients with cancer, and contrasts aspects of the molecular diagnostic
lab with characteristics of the traditional lab.
Molecular Dx helps individualize medicine
Molecular diagnostics is a new discipline that uses
cutting-edge technologies, such as gene chips and microarrays, to obtain
information about the activity patterns of genes and proteins in normal
cells and in cancer cells.1 Researchers use this information to
design better treatments and to identify subgroups of patients with cancer
types that make them likely to respond to specific therapies. Contrast this
approach with the practice of medicine before molecular diagnostics when
cancer cells were examined under a microscope and categorized by their
appearance.
As more tests are validated in the clinic, there is
an increased demand for patient-specific information and an increased
acceptance of the value of molecular diagnostics by regulatory agencies as
well as clinicians. It is not surprising that molecular diagnostic testing
is the fastest growing segment of the in vitro diagnostics (IVD)
market with an estimated annual growth rate of 11%. In 2007, the molecular
diagnostics market totaled $3.21 billion in revenues and is projected to
reach $5.42 billion by 2012.2
This ability to diagnose the molecular basis or the
genetic components of a disease has advanced the concept of “individualized
medicine” beyond finding the right drug at the right dose for the right
patient. Today, clinicians rely on molecular test results to diagnose
disease, and choose therapies most likely to be effective for a specific
tumor and a specific patient.
Molecular Dx improves the management of cancer
In 2008, cancer specialists used the information
provided by molecular diagnostic tests to identify individuals at
increased risk, to enhance the accuracy of diagnoses, and to
individualize treatment decisions for a variety of cancers including two
of the most common forms: breast cancer and colorectal cancer.
Meanwhile, other uses of these tests are being
validated in the clinic. One important use, particularly for oncologists, is
the ability to use the information to predict the course of a disease making
these tests “prognostics” as well as diagnostics.
Breast cancer: Approximately 5% to 10%
of the more than 192,000 American women diagnosed each year with breast
cancer have a hereditary form of the disease. Inherited alterations in the
genes called BRCA1 and BRCA2 are involved in many cases of hereditary breast
and ovarian cancer. Women with an altered BRCA1 or BRCA2 gene are three to
seven times more likely to develop breast cancer than women without
alterations in those genes. This information enables patients from high-risk
families to intervene before cancer occurs.
Of the women diagnosed with breast cancer, 25% have
tumors that overexpress human epidermal growth factor receptor 2 (HER-2).
These tumors are particularly aggressive and have a high risk of recurrence.
A monoclonal antibody, trastuzumab (Herceptin), was found to be most
effective in treating patients with HER-2 positive tumors.3
Fluorescence in situ hybridization (FISH) is a molecular test used to
determine the tumor’s HER-2 status. In fact, the Food and Drug
Administration (FDA)-approved use of Herceptin is the adjuvant or
combination treatment of women with HER-2 overexpressing breast cancer.3
Colorectal cancer: Colorectal cancer is
the second most common cancer in North America. Following surgery, accurate
tumor staging is the most important tool for determining treatment and
prognosis.4,5 Patients with disease limited to the colon or
rectum may have no further treatment; but once cancer has spread to the
adjacent lymph nodes, additional treatment will be recommended.
When traditional histopathology fails to visualize
cancer cells in regional lymph nodes, it is possible the tumor has not
metastasized — but it is also possible cancer cells were not seen in the
section of the lymph node examined. In fact, colorectal cancer will recur in
25% to 30% of patients thought to have negative lymph nodes based on
examination by traditional histopathology.4
In 2008, a molecular test became commercially
available, which is used to thoroughly examine lymph nodes for the presence
of guanylyl cyclase C (GCC), a transmembrane receptor protein specifically
expressed in the lumen of the gastrointestinal tract6 using a
quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR).
In patients with negative lymph nodes by traditional
histopathology, the detection of GCC in a regional lymph node is consistent
with spread of cancer outside the colon or rectum.7 This
information enables clinicians to predict which patients are likely to
experience a recurrence and should receive additional therapy.
Once it is determined patients with colorectal cancer
require additional therapy, molecular diagnostic testing of their tumor
tissues provides important information to optimize the choice of
chemotherapeutic agents.
ResponseDX: Colon (Response Genetics Inc., Los
Angeles, CA), includes three molecular tests used together to “personalize”
cancer chemotherapy for an individual patient with colorectal cancer. The
patient’s own tumor tissue is tested for ERCC1 and for TS (thymidylate
synthetase) expression using qRT-PCR and PCR is used to analyze the DNA to
determine K-ras gene (KRAS) mutational status.
A better response to 5-FU and oxiplatin (FOLFOX) is
seen with low expression of ERCC1 and with low expression of TS. KRAS
mutations occur in approximately 40% of colorectal cancers and confer
resistance to treatment with epidermal growth factor receptor
(EGFR)-targeted therapies such as Erbitux (cetuximab) and Vectibix (panitumumab).8
The National Comprehensive Cancer Network (NCCN) amended its guidelines this
year to recommend KRAS mutation status be determined for all patients
diagnosed with metastatic colorectal cancer. Since the presence of KRAS
mutation strongly predicts a lack of response as well as shorter survival
from EGFR-directed chemotherapy, only patients with metastatic colorectal
cancer who have normal KRAS status should receive anti-EGFR
therapies.
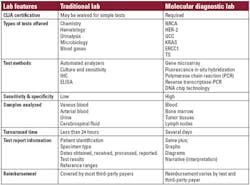
Traditional lab vs. molecular lab: Acute care meets disease management
Due to the growing importance of molecular testing, it is of interest to
compare and contrast a traditional lab with a molecular diagnostic lab. In
simplistic terms, a traditional lab provides information needed by
clinicians in an acute care setting while molecular diagnostic labs provide
genomic and proteomic information to guide the management of chronic
diseases like cancer. Key characteristics of each type of lab are summarized
here and in Table 1.
laboratories
Accreditation required: The United
States Congress enacted the Clinical Laboratory Improvement Amendments
(CLIA) of 1988 to ensure the accuracy and reliability of all laboratory
testing. The CLIA statute is based on the complexity of tests performed and
includes all types of testing sites. Simple tests with a small chance of
error or risk, may be exempt from CLIA rules and are referred to as “waived
test.” Depending on the types of testing offered, a traditional lab may be
required to secure CLIA certification. For all molecular diagnostic testing
sites, CLIA certification is mandatory.
Tests offered and test methods: In a
traditional laboratory, automated analyzers perform the frequently requested
chemistry and hematology tests. Reference ranges and standards are based on
populations. In contrast, molecular testing is highly complex and includes
fluorescence in situ hybridization, polymerase chain reaction (PCR),
reverse transcription-PCR (RT-PCR), DNA chip technology, and gene
microarrays. The genetic information from an individual is analyzed and the
results are unique to each patient. Unlike some of the tests performed at
the traditional labs, molecular diagnostic tests are not routinely used for
surveillance nor are they repeated every few weeks or months.
Sensitivity and specificity: In
contrast to the tests performed in the traditional laboratory, molecular
diagnostic tests need to be both highly sensitive and highly specific
because patients are diagnosed and treatments are administered based on the
results of these tests. A false positive or a false negative might result in
a patient not getting the appropriate treatment. Although no tumor marker is
present in all tumors and none is 100% specific to a tumor, PCR is more
sensitive and specific than protein-based tests such as immunohistochemistry
(IHC) and enzyme-linked immunosorbent assay (ELISA) offered in traditional
labs.
Samples analyzed: A traditional
laboratory receives samples of fresh or frozen
human body fluids for testing. In comparison, a
molecular diagnostic lab may receive fresh tissues or fixed specimens
obtained from bone marrow, lymph nodes, and tumors. New tests are performed
using formalin-fixed, paraffin-imbedded (FFPE) lymph nodes and tumor
tissues, respectively.
Turnaround time: Traditional labs that
perform routine analyses (e.g., chemistry and hematology) can often provide
results in less than one hour. This is due to automation in response to
sample volume, price competition, and demand for rapid turnaround. For
complex multistep molecular tests, there is currently less demand for rapid
turnaround, however, most molecular tests can be completed within several
days.
Test reports: The traditional lab
report includes patient information as well as test results and normal
ranges with minimal, if any, information on the interpretation or the
clinical significance of the values reported. Due to the complexity of
molecular diagnostic tests, interpretation within the context of the disease
or disorder as well as the test methodology for each individual patient is
provided for the ordering clinician. This information may be provided with
graphics as well as in narrative form.
Reimbursement: The tests provided by
traditional labs are covered by most third-party payers although price
competition has led some insurers to restrict patients’ choice of covered
laboratories. For molecular diagnostics, reimbursement must be secured from
third-party payers for each individual test. Molecular labs must work with
individual insurance plans to provide the types of information required.
A new frontier for laboratories
Cutting-edge technologies based on genomics and
proteomics have given rise to a new discipline of molecular diagnostics
which, in turn, has changed the landscape of the traditional laboratory.
Molecular diagnostic labs offer complex tests with results based on the
genetic make-up of the tissues from an individual patient and provide
information in their test reports to aid in the interpretation of the
results to assist clinicians.
Clinicians rely on information from molecular
diagnostic tests to guide treatment decisions for individual patients, while
the FDA uses information from these tests to approve new treatments for
patients most likely to benefit.
The high complexity of the tests performed in the
molecular diagnostic laboratory, the need for interpretation of the test
results for each individual patient, and the disease setting in which the
information is used are some of the key differences in comparison to
traditional laboratory.
Marilyn R. Carlson, DMD, MD, RAC, is affiliated with entreMeDica in Encinitas, CA.
References
- Understanding Cancer Series: Molecular Diagnostics.
www.cancer.gov/cancertopics/understandingcancer/moleculardiagnostics.
Accessed January 1, 2009. - IVD Technology E-Newsletter, November 2008.
- Herceptin (trastuzumab). (Full Prescribing Information.) Genentech.
Revision Date: May 2008 . - Iddings D, Ahmad A, Elashoff D, Bilchik A. The prognostic effect of
micrometastases in previouslystaged lymph node negative (N0) colorectal
carcinoma: a meta-analysis. Annals of SurgicalOncology.
2006;11:1386-1392. - Compton CC, Fielding P, Burgart LJ, et al. Prognostic factors in
colorectal cancer: College ofAmerican Pathologists Consensus Statement
1999. Arch Pathol Lab Med. 2000;124:979-994. - Birbe R, Palazzo JP, Walters R, et al. Guanylyl cyclase C is a
marker of intestinal metaplasia,dysplasia and adenocarcinoma of the
gastrointestinal tract. Hum Pathol. 2005;36:170-179. - Frick GS, Pitari GM, Weinberg DS, Hyslop T, Schulz S, Waldman SA.
Guanylyl cyclase C: amolecular marker for staging and postoperative
surveillance of patients with colorectal cancer. Expert Rev Mol Diagn.
2005;5:701-713. - Karapetis CS et al. K-ras mutations and benefit from
cetuximab in advanced colorectal cancer. N Engl J Med.
2008;359:1757-1765
Contest offers organizational help for the messy office
Again this year, Lab
Quality Confab and Ascendium Consulting are sponsoring “The (Dis)Organization
‘LEAN’ Contest” to help one lab professional straighten up that messy
desk once and for all. The winner receives a daylong date with LEAN
expert Caroline Ambrose, managing consultant for Ascendium, which
provides an office-space makeover, based on the lucky winner’s
organizational type, and ideas to help sustain an orderly workspace
long-term. While lab supervisors might do a great job implementing LEAN
principles on the workbench, their desk areas often are not up to par,
says Ambrose. LEAN means “Everything has a place.” An organized office
is not just for show, she notes. Time lost trying to locate papers is
wasting a valuable resource. “With unannounced inspections, how much
time would staff waste looking for a piece of paper if it was needed for
an inspection when you are absent?”
Last year’s contest winner, Ina Aiazzone, manager of
laboratory quality management and POCT at St. Joseph’s Healthcare System,
Patterson, NJ, is a “piler.”
“Every time someone needed a document, it took me 15
minutes to find it.” she says. Aiazzone maintains desktop order by now using
that 15 minutes to sort and discard unneeded materials on her desk. An
out-basket collects everything that belongs somewhere else, which Aiazzone
delivers to their destinations at the end of the day. She uses five project
boxes with lids to collect everything related to current projects. Her lab
is currently going through a state licensing process, she says. “Now, rather
than piles of paper, anything pertaining to the state license is in a
container where I can always find it.”
Anyone attending 2009 Lab Quality Confab,
September 29-30 in Atlanta is eligible to enter this contest. A contestants
should e-mail name, organization, job title, mailing address, and phone
number, along with a picture of the desktop in question, and explain in 50
words or less why he/she deserves to win. Entries must be received before
midnight. Thursday, Sept. 11. The winner will be announced at 2009 Lab
Quality Confab.