Laboratory professionals can help reverse the rising trend in sexually transmitted infections
Every healthcare professional experienced the strain that the SARS-Cov-2 pandemic exerted across all aspects of patient care. In the fight against sexually transmitted infections (STIs), the sudden disruption to routine screening and diagnostic testing highlighted what we already knew—that when these services are limited, STIs thrive.
In the United States, with access to state-of-the-art testing technologies and highly skilled laboratory professionals, the spread of STIs should be decreasing. Unfortunately, the opposite is happening. In April 2022, the Centers for Disease Control and Prevention (CDC) released its Sexually Transmitted Diseases (STD) Surveillance report. Although this report revealed that cases of STDs fell sharply in 2020, this was due to early COVID-19 prevention strategies and the resulting temporary halt of routine sexual healthcare. As the pandemic evolved, and patients and clinicians returned to the exam room and testing sites, STI diagnoses surged back to record levels by the end of the year.1
Infection rates of Neisseria gonorrhea (NG) and primary and secondary (P&S) syphilis have reached historic highs in the United States year-after-year since 2016. Cases of NG have increased 111% since an all-time low in 2009 and rose 46% between 2016 and 2020.1 In part, these increases are the result of access to new, highly sensitive diagnostic tests for detecting STIs. At the same time, the challenge to combat infections through screening remains aggravated by economic and racial disparities. In 2020, for instance, non-Hispanic black persons represented an estimated 32% of all cases of Chlamydia trachomatis (CT), NG, and primary and secondary (P&S) syphilis despite being just approximately 12% of the U.S. population.1
These data are based on screening coverage for less than 60% of the population at greatest risk for STIs — women ages 15–24. 2 Further, reduced access to sexual health services due to COVID-19 left gaps in surveillance data for the year 2020, which limited tracking by the CDC of STIs in general. This included tracking antimicrobial resistant strains of many organisms, for instance, antibiotic-resistant NG, which is essential information for formulating national STI treatment guidelines.3 Although reports of CT infection have fallen in recent years (down 1.2% from 2016 to 2020 and 13% between 2019 and 2020),1 the CDC notes that these statistics are “unlikely due to a reduction in new infections” because most CT infections are asymptomatic and can only be detected through proactive, routine screening. Even if the numbers are trending downward, CT infections reported to the CDC in 2020 were nearly 1.6 million — within the limited surveillance coverage.1
While the CDC urges caution interpreting its 2020 Surveillance report, there is no doubt that the COVID-19 response significantly affected STI screening, testing, and mitigation efforts, and it is unclear how the pandemic will affect the future prevalence of STIs and STI-associated adverse health outcomes. Intriguingly, one thing the available data show is that in the fight to reverse these alarming trends in common bacterial STIs, laboratory professionals can play an essential role, by both educating and supporting healthcare providers.
Opt-out screening: A straightforward solution to a delicate problem
One difficulty providers face is that because more than half (53%) of all STIs occur in adolescents and young adults (ages 15–24), this includes many patients who may be reluctant to discuss their sexual health. This obstacle is acutely troublesome when females in this age group account for approximately 76% of all CT and 58% of all NG infections, and 84% of patients infected with either STI will be asymptomatic.4,5
Undiagnosed and untreated CT/NG infections are associated with serious adverse effects, including pelvic inflammatory disease (PID), infertility, and ectopic pregnancies, and the current rate of CT/NG infection is an unacceptable 5.5 million new cases per year.4 To address this challenge, the CDC now recommends that healthcare providers consider an opt-out approach to CT/NG screening for adolescent and young adult females, which offers screening without asking patients about their recent sexual behavior.6
Opt-out screening is endorsed by the American Sexual Health Association – National Chlamydia Coalition, which provides support and educational materials on the subject to healthcare providers.7 If widely adopted, this approach would help achieve the goal outlined in the 2021–2025 National STI Strategic Plan to increase routine screening for these common STIs in this population from 59% to 77% over a 10-year period.8
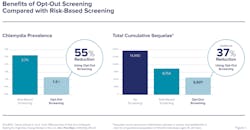
A comparison of opt-out screening to risk-based testing in one study found that the former was shown to reduce total prevalence of STI infection by an additional 55% and achieved an additional 37% reduction in total cumulative sequelae (Figure 1). The study’s authors stated that “substantially high test acceptance probabilities (>95%) are achievable” notwithstanding health insurance and other barriers to accessing healthcare.9 Additionally, researchers at the University of Pittsburgh School of Medicine found that 62% of parents of adolescents would consent to routine CT/NG screening of their children.10
To support initiatives like opt-out screening, as well as other goals to mitigate STIs, laboratories can align their test offerings with current guidelines. FDA-cleared nucleic acid amplification tests (NAAT) using vaginal swab specimens are recommended by the CDC for routine CT/NG screening for all females. Screening is also recommended for sexually active young males in clinical settings with a high prevalence of CT and/or NG by means of first void urine samples and FDA-cleared NAAT assays.6 Laboratories and healthcare providers who follow these guidelines can help limit the spread of these and other STIs.
The most common STI should not be so common
The most prevalent nonviral STI worldwide, the protozoan Trichomonas vaginalis (TV), affects 3.7 million persons in the United States each year. Unlike CT/NG, TV infection is found equally among patients under and above 24 years old11 and infection rates are higher in females than in males.12 Often associated with having new or multiple sex partners, untreated TV may lead to reproductive morbidities, including a 1.4 times greater likelihood of preterm birth, premature rupture of membranes, and undersize infants for their gestational age.13 This STI is also associated with a 1.5 times greater risk of HIV acquisition and is associated with an increase in HIV vaginal shedding in those who are living with HIV, but who are not virally suppressed, according to the CDC.14
Fortunately, TV is highly treatable — when it is detected. Testing for TV is recommended for all females presenting with vaginal discharge, and annual screening should be considered for both females and males in high-prevalence settings (e.g., sexual health clinics and correctional facilities). It should also be considered for asymptomatic females at high risk for infection (e.g., multiple sex partners, transactional sex, drug misuse, or a history of STIs or incarceration.) Routine annual screening for TV among asymptomatic females with HIV is recommended. FDA-cleared NAAT assays are preferred for accuracy, as these tests detect TV three–five times more consistently than wet-mount microscopy.15,16
Detecting tiny troublemakers like Mycoplasma genitalium
While we must refocus efforts on common STIs like NG, CT, TV, and P&S syphilis, laboratories will become more integral partners as we confront emerging STI pathogens that are less well known, even to specialists in sexual and reproductive health. The tiny microbe Mycoplasma genitalium (M. genitalium) lacks a cell wall and can take months to culture,17 but this elusive STI is associated with urethritis in males and PID, preterm delivery, spontaneous abortion, and infertility in females, and some data also indicate an association between M. genitalium and other STIs, including HIV.6
Detected in both males and females at rates that are consistently higher than NG and often higher than CT, M. genitalium was listed in 2015 by the CDC as an “emerging issue” when the lack of a practical testing protocol precluded any clear guidelines. But after the first FDA-cleared NAAT assay for M. genitalium was introduced to the market in 2019,18 the CDC updated its 2021 guidelines to recommend testing for M. genitalium in females with persistent cervicitis and men with recurring urethritis, and now recommends testing should be considered for females with PID.6
When M. genitalium infection is suspected, it is essential that it be identified and not misdiagnosed as a different STI because the treatment is critically distinctive. FDA-cleared NAAT assays for M. genitalium are the CDC-recommended diagnostic method, as culture methods are protracted and require specialized laboratory capacity.17 Laboratories that are both equipped for and informed about M. genitalium guidelines can help healthcare providers identify and target the populations most likely to be affected by this troublesome pathogen.
Guided by the most current CDC recommendations and national strategic initiatives, healthcare providers and laboratories alike can work together to mitigate, and ultimately reverse, the growing rate of STI transmission in the United States. The COVID-19 pandemic revealed many shortcomings in our healthcare system. Nevertheless, the laboratory professionals who adopt the most up-to-date test offerings and surveillance recommendations for STIs can support and even lead healthcare providers to make smart, efficient, and cost-effective decisions to improve patient care.
References
- Sexually Transmitted Disease Surveillance 2020. CDC. https://www.cdc.gov/std/statistics/2020/default.htm. Accessed August 29, 2022.
- DiClemente RJ, et al. Association between sexually transmitted diseases and young adults’ self-reported abstinence. Pediatrics. 2011;127(2):208-13. doi:10.1542/peds.2011-0295.
- COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. CDC. https://stacks.cdc.gov/view/cdc/117915. Accessed August 29, 2022.
- Kreisel KM, Spicknall, IH, Gargano, JW, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48(4):208-214. doi:10.1097/OLQ.0000000000001355.
- Detels R, Green AM, Klausner JD, et al. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis. 2011;38(6):503-509. doi:10.1097/olq.0b013e318206c288.
- Workowski KA, Bachmann LH, Chan PA, et al., Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187. doi:10.15585/mmwr.rr7004a1.
- Opt-out screening, ASHA/NCC, http://chlamydiacoalition.org/opt-out-screening/. Accessed August 21, 2022.
- Sexually Transmitted Infections National Strategic Plan for the United States: 2021–2025. US DHHS. https://www.hhs.gov/sites/default/files/STI-National-Strategic-Plan-2021 2025.pdf#:~:text=STI%20National%20Strategic%20Plan%3A%202021%E2%80%932025%20i%20The%20United,all%20people%2C%20regardless%20of%20age%2C%20sex%2C%20gender%20identity%2C. Accessed August 29, 2022.
- Owusu-Edusei K Jr, Hoover KW, Gift TL. Cost-effectiveness of opt-out chlamydia testing for high-Risk young women in the U.S. Am J Prev Med. 2016;51(2):216-224. doi:10.1016/j.amepre.2016.01.007.
- Lane KC, et al. Many parents would accept sexually transmitted infection screening for their adolescent at a pediatric office visit. J Adolesc. Health. 2020;66(5):626-628. doi:10.1016/j.jadohealth.2019.12.019.
- Flagg EW, et al. Prevalence of Trichomonas vaginalis among civilian, noninstitutionalized male and female population aged 14 to 59 years: United States, 2013 to 2016. Sex Transm Dis. 2019;46(10):e93-e96. doi:10.1097/olq.0000000000001013.
- Muzny CA, et al. Added benefit of nucleic acid amplification testing for the diagnosis of Trichomonas vaginalis among men and women attending a sexually transmitted diseases clinic. Clin Infect Dis. 2014;59(6):834-841. doi:10.1093/cid/ciu446.
- Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a cause of perinatal morbidity: A systematic review and meta-analysis. Sex Transm Dis. 2014;41(6):369-376. doi:10.1097/olq.0000000000000134.
- Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183(7):1017-1022. doi:10.1086/319287.
- Hollman D, Coupey SM, Fox AS, Herold BC. Screening for Trichomonas vaginalis in high-risk adolescent females with a new transcription-mediated nucleic acid amplification test (NAAT): associations with ethnicity, symptoms, and prior and current STIs. J Pediatr Adolesc Gynecol. 2010;23(5):312-316. doi:10.1016/j.jpag.2010.03.004.
- Roth AM, Williams JA, Ly R, et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38(5):398-400. doi:10.1097/OLQ.0b013e318203e3ce.
- Jensen JS, Hansen HT, Lind K. Isolation of Mycoplasma genitalium strains from the male urethra. J Clin Microbiol. 1996;34(2):286-291. doi:10.1128/jcm.34.2.286-291.1996.
- Gaydos CA, Manhart LE, Taylor SN, et al. Molecular Testing for Mycoplasma genitalium in the United States: Results from the AMES prospective multicenter clinical study. J Clin Microbiol. 2019;57(11). doi:10.1128/JCM.01125-19.
About the Author

Jennifer Balkus, PhD, MPH
is an Associate Professor at the University of Washington School of Public Health in Seattle. As an infectious disease epidemiologist, her research spans across the fields of sexual and reproductive health. She is passionate about improving genital health for all people with a vagina and has contributed to the 2021 Centers for Disease Control and Prevention STI guidelines for the treatment of bacterial vaginosis. She has expertise in conducting both clinical trials and observational studies, in the US and globally, and currently leads two NIH R01-supported research projects. The first is evaluating the relationship between vaginal bacterial and susceptibility to Chlamydia trachomatis infection among a cohort of cisgender women in Kenya. The second project is assessing opportunities to improve STI testing and treatment to reduce STI recurrence among a cohort of cisgender adolescent girls and young women in South Africa.

Christina A. Muzny, MD, MSPH
is a tenured Associate Professor in the Division of Infectious Diseases at University of Alabama at Birmingham (UAB) with secondary appointments in the UAB Department of Epidemiology and Obstetrics & Gynecology. She obtained her medical degree at the Texas A&M University Health Sciences Center College of Medicine followed by an internal medicine residency and an infectious diseases fellowship at the University of Mississippi Medical Center. She has since obtained an MSPH in Public Health (Epidemiology) at the UAB School of Public Health. Her clinical and research interests focus on the HIV and STIs in women, specifically vaginal infections including BV and trichomoniasis. Dr. Muzny currently has R01 and R21 funding from NIH/NIAID to study the pathogenesis of BV.