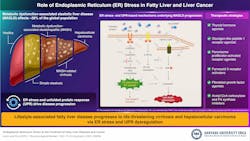
Endoplasmic reticulum (ER) stress and the unfolded protein response are often implicated in the progression of metabolic dysfunction-associated steatotic liver disease (MASLD), from simple steatosis to inflammation-driven metabolic dysfunction-associated steatohepatitis and, ultimately, hepatocellular carcinoma.
A new review paper from Hanyang University ERICA sheds light on the mechanisms underlying these processes, advocating for precise hepatic ER stress control to maintain normal hepatocyte function and develop therapeutic strategies against MASLD.
Nonalcoholic fatty liver disease, now re-termed metabolic dysfunction-associated steatotic liver disease (MASLD), affects nearly 30% of the global population. It is strongly linked to endoplasmic reticulum (ER) stress. The ER is a dynamic membranous organelle that constitutes about half of the membrane content in liver cells and acts as the main hub for protein folding and lipid biosynthesis. Notably, the unfolded protein response (UPR) is a key mechanistic stress regulator that influences disease progression from simple steatosis to inflammation-driven metabolic dysfunction-associated steatohepatitis (MASH) and eventually hepatocellular carcinoma—the third leading cause of cancer-related mortality worldwide.
In a new review article, an international team of researchers—led by Associate Professor Ju Youn Kim from the Major in Molecular Medicine, School of Bio-Pharmaceutical Convergence, Hanyang University ERICA, Republic of Korea—has comprehensively investigated how ER stress and the UPR drive the progression of MASLD from simple fatty liver to MASH and liver cancer.
The comprehensive analysis examines therapeutic strategies targeting ER stress pathways, including recently approved GLP-1 receptor agonists, and explores the brain-liver connection in metabolic disease progression. The article further highlights newly identified molecular mechanisms underlying the progression of advanced MASH to MASH-related cirrhosis and hepatocellular carcinoma. In particular, the team focuses on the role of hepatic ER stress in hepatocyte injury and stress responses within a lipid-overloaded liver tissue context.
This work presents a holistic framework for understanding ER-mediated metabolic outcomes and can guide future research on the development of novel therapeutic strategies.