Molecular testing ushers in a new era of rapid diagnostics for pharyngitis
Acute pharyngitis “sore throat” is an inflammatory condition of the pharynx and/or tonsils commonly observed in both adults and children. Viruses are primarily responsible, but bacteria are also implicated. Infection with beta-hemolytic Streptococcus pyogenes, or Group A streptococcus (GAS), accounts for 5%–15% and 20%–30% of infections in adults and children worldwide, respectively. Acute pharyngitis is one of the most common reasons for primary care visits1 and is the most common diagnosis linked to antibiotic use in school-aged children.2 Antibiotics are ineffective against viral pharyngitis and do not shorten illness duration or improve patient outcomes. Because throat culture takes up to 48 hours to produce actionable results, clinicians may preemptively prescribe antibiotics “just in case” the infection is due to GAS. This practice leads to unnecessary antibiotic use and the promotion of bacterial resistance. According to a recent study, it is estimated that nearly half of antibiotic prescriptions for pharyngitis are unnecessary because most infections are of viral origin.3 This practice also wastes healthcare resources and unnecessarily subjects patients to antibiotic-associated side effects. Moreover, other pathogenic bacteria may be responsible for the infection and these may not be responsive to conventional GAS therapy. Rapid, accurate, and reliable testing solutions are needed to provide timely patient information during the clinician office visit. State-of-the-art nucleic acid amplification tests (NAAT) can fulfill this need and have the potential to improve antimicrobial stewardship.3 This article will address the complexities of acute pharyngitis diagnosis and treatment and summarize emerging clinical data pointing to the advantages of NAAT over present testing recommendations.
Present testing guidelines for pharyngitis
Current guidelines for the diagnosis and management of GAS pharyngitis were released by the Infectious Diseases Society of America (IDSA) in 2012.4 For children ≥ 3 years of age, testing should consist of a rapid antigen detection test (RADT) or throat culture.4 Due to variable sensitivity of RADTs, throat culture is recommended for children and adolescents with a negative RADT. Due to the high specificity of RADTs, throat culture is not recommended for a positive RADT.4 Furthermore, the IDSA advises against routine GAS testing for patients <3 years of age as the incidence of GAS pharyngitis and rheumatic fever are rare in this population.4,5 The IDSA also advises against GAS testing in individuals without pharyngitis or pharyngitis associated with viral symptoms to prevent false positive results due to GAS colonization.4,5 Viral symptoms include cough, runny nose, hoarseness, oral ulcers, conjunctivitis, and/or diarrhea.4 Testing outside of these recommendations leads to spurious test results, misdiagnoses, medication side effects, and promotion of antibiotic resistance.6-10 Nearly three decades ago, it was noted that approximately 70% of patients with sore throats seen in the primary care setting received antibiotic prescriptions.11 Recently, a retrospective cohort study found that nearly 40% of pediatric patients tested for GAS were not compliant with IDSA guidelines.5 This translated into greater return rates for patients, misdiagnoses, inappropriate antibiotic use, allergic reactions and loss of school days.5
Antigen and culture testing
The gold standard test for pharyngitis, developed over 70 years ago, is throat culture for the isolation of beta-hemolytic GAS on sheep blood agar.12,13 Culture has a turnaround time of 24 to 48 hours, which markedly delays timely diagnosis and patient management. Despite being the gold standard, culture is not without limitations. The quality of specimen collection is critical for optimal test results. A study investigating dual throat swab collection found that utilization of a single swab would have missed 9% to 12% of positives.14 Following specimen collection, throat swabs should be placed into transport media (e.g., Amies) and expeditiously delivered to the laboratory. Transportation delays >24 hours decrease bacteria viability and increase the chance of false negative test results. Technical expertise is required of laboratory personnel to appropriately cultivate and identify GAS. More importantly, culture does not have the ability to distinguish between infection and colonization. As such, cultivating GAS should never clinically equate to active infection.
Rapid antigen diagnostic tests (RADTs) for GAS were developed over 40 years ago for use at the point-of-care (POC) or within clinical laboratories.15 Various RADT formats are available for use, including latex agglutination, lateral flow immunoassay, and optical immunoassay.13 GAS RADT specificity is approximately 95%. As such, culture for positive RADTs is not warranted. However, GAS RADT sensitivity is insufficient for stand-alone testing. Systemic reviews and meta-analyses estimate the GAS RADT pooled sensitivity at 85% (range 70%–90%).4,16-18 As such, culture is recommended for children and adolescents with a negative RADT result. RADTs also have limitations. A poorly collected sample may contain suboptimal quantities of GAS, which decreases test sensitivity and leads to false negative test results. Some RADTs allow the throat swab to be placed into liquid transport media so that culture can be performed if the RADT is negative. This workflow dilutes the concentration of GAS leading to decreased test sensitivity and false negative RADT results. RADTs rely upon the operator to observe and properly interpret the presence/absence of agglutination, color changes, and/or the presence of detection lines in test strips.13 These human-related tasks introduce intra- and inter-operator bias and inconsistent test results. Bias is further compounded when considering each operator’s visual acuity and degree of color blindness (if present), and the quality of ambient lighting where testing is performed. Some manufacturers have employed optical reading devices to mitigate human interpretative bias. An overlooked limitation of RADTs is their inability to distinguish between colonization and active or past infection. By design, RADTs detect one or more GAS-specific bacterial proteins. A mere positive result does not always equate to active infection. Lastly, at present, commercially available pharyngitis RADTs only detect GAS.
Molecular testing — Technological advancements and supporting clinical data
Molecular testing solutions have undergone significant improvements over the last few decades. Initially, such testing used a chemiluminescent-labeled DNA probe directed at a GAS-specific ribosomal RNA (rRNA) sequence. When compared to culture, this test yielded excellent sensitivity (95%) and specificity (100%).19 However, slow turnaround times and technical limitations, precluded its use into the POC setting. The next decade witnessed the transition from manual to semi- or fully automated DNA extraction and nucleic acid amplification technologies. Although not suitable for the POC setting, these moderate-to-high complexity tests replaced culture for negative RADTs, greatly reducing the turnaround time for definitive results. A study of 2,050 patients in an urgent care setting with negative GAS RADT results demonstrated excellent test sensitivity (91.4%) and specificity (98.5%) for an isothermal amplification test when compared to culture. The authors’ highlighted the ability to use this test as a quicker alternative to culture for negative GAS RADTs.20 A study of 161 patients with negative GAS RADT results showed exceptional test sensitivity (100%) and specificity (100%) of a PCR test when compared to culture. The turnaround time of PCR was 18.1 hours compared to 45.0 hours for culture.21 In the last decade, further nucleic acid extraction and amplification technology advancements have provided a definitive path to enter the POC arena. These include both rapid real-time PCR and isothermal nucleic acid amplification (iNAA) technologies. In general, iNAA yields faster, more cost-effective test results by negating the need for iterative heating and cooling on an expensive thermocycler — a requirement of real-time PCR. Presently, commercially available iNAA technologies amplify and detect one or two nucleic acid targets. In contrast, high-level multiplexing (≥4 targets) is already available with real-time PCR. Improved multiplexing capabilities is possible with iNAA,22,23 and will likely be a requisite to meet the growing demands of the rapidly expanding molecular POC testing market.
To date, several NAATs have received FDA-approval and CLIA-waived status allowing their use in the POC setting (Table 1). Two recent studies (1,673 symptomatic patients) demonstrated excellent test sensitivity (>96%) and specificity (>93%) for two different iNAA assays when compared to culture.24,25 A smaller study (145 symptomatic patients) demonstrated excellent test sensitivity (100%) and good specificity (79.3%) of a real-time PCR test when compared to culture. Lower molecular test specificity was due to increased detection of GAS that was likely below culture detection threshold.26 Three additional studies (648 symptomatic patients) demonstrated excellent test sensitivity (100%) and good-to-excellent specificity (83.5%–97.4%) of rapid real-time PCR when compared to culture.7,27-28 To expedite accurate diagnosis and reduce unnecessary antibiotic use, the authors recommended replacing RADTs and culture with molecular tests.7 Another group recently recommended that rapid real-time PCR be used as first-line testing for GAS.28 Authors of a recent study, whose laboratories collectively served two pediatric hospitals and eight urgent care centers, noted that molecular testing provided definitive results in a timely manner without the need for back-up culture.29 These tests offer turnaround times ranging from 8 to 60 minutes, and test sensitivity and specificity equivalent to and exceeding that of culture and RADTs, respectively. Prior to deployment in the POC setting, these molecular solutions should be vetted with appropriate stakeholders to ensure turnaround time constraints will not adversely affect staffing and patient flow.Molecular testing considerations and concerns
Several issues need consideration prior to adopting NAATs. First, DNA is a highly stable chemical structure and its presence in the testing environment may lead to false positive test results. Adherence to proper specimen handling, unidirectional workflow, frequent glove changes, and environmental decontamination protocols can essentially eliminate this possibility. The incorporation of negative controls into the testing process can also facilitate detection of DNA contamination. Second, DNA polymerases, the enzymes that amplify DNA, are susceptible to interfering substances that may inhibit the nucleic acid amplification reaction. As such, internal amplification controls must be incorporated into the test system to verify that each test performed as expected. In response, manufacturers have developed fail-safe systems using cassettes with minimal pipetting and operator input to reduce human error. Remote instrument monitoring via the internet by laboratory staff and field service engineers is a reality and aids in the real-time monitoring of test and instrument performance. Despite these technological advancements, other challenges still face molecular testing. Due to improved sensitivity over culture, it may be difficult to distinguish colonization from infection. This is relevant in cases of acute pharyngitis where an individual’s clinical signs and symptoms favor a viral etiology.13 The GAS colonization rate can reach 20%–25% in asymptomatic patient populations. Therefore, molecular tests for GAS should only be used on individuals with clinical signs and symptoms supporting a bacterial infection. Another challenge for NAATs is the inability to distinguish between viable from non-viable bacteria or active from resolved infection. In one study, 20% of patients adequately treated for GAS still tested positive by NAAT between 14–18 days after the initial diagnosis.30 Likewise, NAATs should not be used as a “test-of-cure,” especially within the first 14 days after completion of therapy. If clinically indicated, “test-of-cure” should be performed by culture.
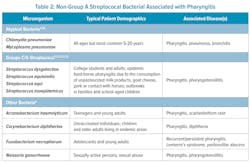
Currently, the IDSA guidelines focus upon the diagnosis and treatment of GAS to prevent extension of infection into the head and neck region and prevent immune-mediated complications (rheumatic fever or kidney damage). However, other pathogenic bacteria may be responsible (Table 2). Certain beta-hemolytic groups C/G streptococci are considered pathogenic and are detected in 3%–22% of pharyngitis cases;31-33 these include S. equi, S. dysgalactiae, S. equisimilis, and S. zooepidemicus.12,21,32Streptococcus dysgalactiae is second only to GAS for prevalence in patients symptomatic for pharyngitis (0.85% in patients younger than 15 years and 2.18% of patients older than 15 years; n = 1799).33 These organisms are primarily seen in college students and adults, and associated with epidemic food-borne pharyngitis.4,34 However, if left untreated, these have not been definitively linked to rheumatic fever or kidney damage.4 Non-streptococcal bacteria have also been implicated in pharyngitis, including Fusobacterium necrophorum, Arcanobacterium haemolyticum, Corynebacterium diphtheriae, Neisseria gonorrheae, Chlamydia pneumoniae, and Mycoplasma pneumoniae. 4,35 Despite having clinical signs/symptoms similar to GAS and available antibiotic therapy, infections due to Groups C/G streptococci and other non-GAS organisms are not frequently tested at the POC (Table 2). Currently, CLIA-waived NAATs for Groups C/G streptococci do not exist. Few CLIA moderate-to-high complexity NAATs are currently available, which largely precludes their use at POC; two of these generate test results in 25 or 60 minutes.36 Non-streptococcus organisms are only detected in laboratories using specialized cultivation, biochemical, latex agglutination, and/or mass spectrometry-based identification techniques. Development of POC multiplex assays for GAS and non-GAS pathogens could improve patient outcomes.Cost and reimbursement are additional considerations. Molecular tests are more expensive than RADTs and culture; however, they do not require expensive, skilled labor. Generally, molecular reimbursement is proportionally higher than RADT and culture. Net revenue, reimbursement minus expense, for NAATs generally provides a viable financial path for molecular testing implementation. Molecular also offers rapid, definitive answers that enhance patient management and provides opportunities to improve antibiotic stewardship.3 Evidence suggests molecular testing results in lower antibiotic usage in individuals with acute pharyngitis.6,8 Likewise, appropriate antibiotic prescribing was enhanced with molecular POC (97.1%) vs. RADT and culture (87.5%).8
Summary
Molecular testing has sufficiently advanced to provide accurate, rapid, and reliable results for the diagnosis of GAS pharyngitis at POC. These user-friendly testing solutions have the potential to improve workflow efficiency, patient satisfaction, clinical outcomes, antibiotic stewardship initiatives, and lower healthcare costs by reducing wait times, return visits, and follow-up calls. In a recent commentary in Clinical Microbiology and Infection, the authors summarized as follows, “[Rapid molecular] tests are here to stay. They are being used now, and they will be used more frequently as clinicians become comfortable with molecular testing for GAS and many other infectious diseases. Published data for the diagnostic accuracy of these tests is growing but it is important that the appropriate clinical context and setting to perform these tests be considered and evaluated.”14 The final frontier for widespread POC molecular testing deployment is speed. The majority of healthcare professionals working in busy outpatient clinics define “rapid” as ≤ 15 minutes (author’s professional opinion); a metric critical for patient management and clinic operational efficiency. Today, a handful of manufacturers offer FDA-approved, CLIA-waived rapid POC molecular testing solutions. Of these, only one generates results in ≤ 10 minutes. Over the next few years, additional POC molecular technological advancements will facilitate enhanced multiplexing capabilities in conjunction with test result generation in 5–10 minutes. We are now entering the ultra-rapid NAAT era.
References
1. Shah M, Centor RM, Jennings M. Severe acute pharyngitis caused by group C streptococcus. J Gen Intern Med. 2007;22(2):272-274. doi:10.1007/s11606-006-0049-4.
2. Vaz LE, Kleinman KP, Raebel MA, et al. Recent trends in outpatient antibiotic use in children. Pediatrics. 2014;133(3):375-85. doi:10.1542/peds.2013-2903.
3. Graf EH. Can rapid molecular Streptococcus pyogenes testing lead to better antimicrobial stewardship for acute pharyngitis? J Appl Lab Med. 2019;4(2):140-142. doi:10.1373/jalm.2019.029983.
4. Shulman, ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Disease Society of America. Clin Infect Dis. 2012;55(10):1279-1282. doi:10.1093/cid/cis847.
5. Thompson JM, Zagel AL, Spaulding AB, Krause EA, Arms JL. Streptococcal pharyngitis: Compliance with national testing guidelines in a pediatric emergency department. Pediatr Emerg Care. 2022;38(2):e519-e523. doi:10.1097/PEC.0000000000002512.
6. Luo R, Sickler J, Vahidnia F, Lee YC, Frogner B, Thompson M. Diagnosis and management of group A streptococcal pharyngitis in the United States, 2011-2015. BMC Infect Dis 2019;19(1):193. doi:10.1186/s12879-019-3835-4.
7. Parker K, Gandra S, Matushek S, Beavis KG, Tesic V, Charnot-Katsikas A. Comparison of 3 nucleic acid amplification tests and a rapid antigen test with culture for the detection of group A streptococci from throat swabs. J Appl Lab Med. 2019;4:(2):164-9. doi:10.1373/jalm.2018.028696.
8. Rao A, Berg B, Quezada T, et al. Diagnosis and antibiotic treatment of group A streptococcal pharyngitis in children in a primary care setting: Impact of point-of-care polymerase chain reaction. BMC Pediatr. 2019;19(1):24. doi:10.1186/s12887-019-1393-y.
9. Tanz RR, Ranniger EJ, Rippe JL, et al. Highly sensitive molecular assay for group A streptococci over-identifies carriers and may impact outpatient antimicrobial stewardship. Pediatr Infect Dis J. 2019;38(8):769-74. doi:10.1097/INF.0000000000002293.
10. Serwecińska L. Antimicrobials and antibiotic-resistant bacteria: a risk to the environment and to public health. Water. 2020; 12(12):3313. https://doi.org/10.3390/w12123313.
11. Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. J Am Med Assoc. 1998;279(11):875-877. doi:10.1001/jama.279.11.875.
12. The evolution of group A streptococcus pharyngitis testing. Aacc.org. Accessed May 18, 2023. https://www.aacc.org/cln/articles/2018/september/the-evolution-of-group-a-streptococcus-pharyngitis-testing.
13. Thompson TZ, McMullen AR. Group A streptococcus testing in pediatrics: The move to point-of-care molecular testing. J Clin Microbiol. 2020;58(6):e01494-19. doi:10.1128/JCM.01494-19.
14. Patel AB, Shulman ST, Tanz RR. Here to stay: Rapid nucleic acid tests for group A streptococcus pharyngitis. Clin Microbiol Infect. 2021;27(12):1718-1720. doi:10.1016/j.cmi.2021.07.037.
15. Mustafa Z, Ghaffari M. Diagnostic methods, clinical guidelines, and antibiotic treatment for group A streptococcal pharyngitis: a narrative review. Front Cell Infect Microbiol. 2020;10:563627. doi:10.3389/fcimb.2020.563627.
16. Ruiz-Aragón J, Rodríguez López R, Molina Linde JM. Evaluación de los métodos rápidos para la detección de Streptococcus pyogenes. Revisión sistemática y metaanálisis [Evaluation of rapid methods for detecting Streptococcus pyogenes. Systematic review and meta-analysis]. Ann Pediatr (Barc). 2010;72(6):391-402. doi:10.1016/j.anpedi.2009.12.012.
17. Lean WL, Arnup S, Danchin M, Steer AC. Rapid diagnostic tests for group A streptococcal pharyngitis: a meta-analysis. Pediatrics. 2014;134(4):771-81. doi:10.1542/peds.2014-1094.
18. Cohen JF, Bertille N, Cohen R, Chalumeau M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst Rev. 2016;7(7):CD010502. doi:10.1002/14651858.CD010502.pub2.
19. Chapin KC, Blake P, Wilson CD. Performance characteristics and utilization of rapid antigen test, DNA probe, and culture for detection of group A streptococci in an acute care clinic. J Clin Microbiol. 2002;40(11):4207-10. doi:10.1128/JCM.40.11.4207-4210.2002.
20. Arbefeville S, Nelson K, Thonen-Kerr E, Ferrieri P. Prospective postimplementation study of Solana group A streptococcal nucleic acid amplification test vs conventional throat culture. Am J Clin Pathol. 2018;30;150(4):333-337. doi:10.1093/ajcp/aqy051. PMID: 29982326.
21. Boyanton BL Jr, Darnell EM, Prada AE, Hansz DM, Robinson-Dunn B. Evaluation of the Lyra Direct Strep Assay to detect group A streptococcus and group C and G beta-hemolytic streptococcus from pharyngeal specimens. J Clin Microbiol. 2016;54(1):175-7. doi:10.1128/JCM.02405-15.
22. Tong Y, Tang W, Kim HJ, Pan X, Ranalli TA, Kong H. Development of isothermal TaqMan assays for detection of biothreat organisms. Biotechniques. 2008;45(5):543-557. doi:10.2144/000112959.
23. Tanner NA, Zhang Y, Evans TC Jr. Simultaneous multiple target detection in real-time loop-mediated isothermal amplification. Biotechniques. 2012;53(2):81-9. doi:10.2144/0000113902.
24. Faron ML, Ledeboer NA, Granato P, et al. Detection of group A Streptococcus in pharyngeal swab specimens by use of the AmpliVue GAS isothermal helicase-dependent amplification assay. J Clin Microbiol. 2015;53(7):2365–2367. doi:10.1128/JCM.00687-15.
25. Cohen DM, Russo ME, Jaggi P, Kline J, Gluckman W, Parekh A. 2015. Multicenter clinical evaluation of the novel Alere i strep A isothermal nucleic acid amplification test. J Clin Microbiol. 2015;53(7):2258-2261. doi:10.1128/JCM.00490-15.
26. Ralph AP, Holt DC, Islam S, et al. Potential for molecular testing for group A streptococcus to improve diagnosis and management in a high-risk population: A prospective study. Open Forum Infect Dis. 2019;26;6(4):ofz097. doi:10.1093/ofid/ofz097.
27. Ferrieri P, Thonen-Kerr E, Nelson K, Arbefeville S. Prospective evaluation of Xpert® Xpress Strep A automated PCR assay vs. Solana® Group A streptococcal nucleic acid amplification testing vs. conventional throat culture. Curr Microbiol. 2021;78(8):2956-2960. doi:10.1007/s00284-021-02547-0.
28. Taylor A, Morpeth S, Webb R, Taylor S. The utility of rapid group A streptococcus molecular testing compared with throat culture for the diagnosis of group A streptococcal pharyngitis in a high-incidence rheumatic fever population. J Clin Microbiol. 2021;59(12):e0097821. doi:10.1128/JCM.00978-21.
29. Weinzierl EP and Gonzalez MD. you say that you want a molecular revolution? Changing from the group A streptococcus antigen and culture paradigm to molecular testing. Clin Microbiol Newsletter. 42;(13):105-110. doi:10.1016/j.clinmicnews.2020.06.001.
30. Homme JH, Greenwood CD, Cronk LB, et al. Duration of Group A streptococcus PCR positivity following antibiotic treatment of pharyngitis. Diagn Microbiol Infect Dis. 2018;90:105-8. doi:10.1016/j.diagmicrobio.2017.10.003.
31. Jose JJM, Brahmadathan KN, Abraham VJ, et al. Streptococcal group A, C and G pharyngitis in school children: A prospective cohort study in Southern India. Epidem Infect. 2018;16(7):848-853. doi:10.1017/S095026881800064X.
32. Kaplan EL, Gerber MA. Group A, Group C, and Group G Beta-Hemolytic Streptococcal Infections. (Feigin RD, Cherry J, Demmler-Harrison GJ, Kaplan SL, eds.). Elsevier; 2009.
33. Dawson P, Malone L, Grigorenko E. Prevalence of Streptococcus dysgalactiae and microbial codetection in patients presenting with pharyngitis. Open Forum Infect Dis. 2017;4(suppl_1):S586. doi:10.1093/ofid/ofx163.1536.
34. Zaoutis T, Attia M, Gross R, Klein J. The role of group C and group G streptococci in acute pharyngitis in children. Clin Microbiol Infect. 2004;10(1):37-40. doi:10.1111/j.1469-0691.2004.00732.x.
35. Centor RM, Geiger P, Waites KB. Fusobacterium necrophorum bacteremic tonsillitis: 2 case and a review of the literature. Anaerobe. 2010;16:626-8. doi:10.1016/j.anaerobe.2010.07.008
36. Gonzalez MD, McElvania E. New developments in rapid diagnostic testing for children. Infect Dis Clin North Am. 2018;32(1):19-34. doi:10.1016/j.idc.2017.11.006.
About the Author

Jane M. Caldwell, PhD
is the executive director of the medical education company Medavera, Inc. and has over 25 years of diverse experience with research and development in multiple areas of epidemiology and molecular biology. She has published extensively in peer-reviewed journals, book chapters, and the popular press. Dr. Caldwell recently worked as a consultant to troubleshoot algorithms for qPCR detection of COVID-19 infections which improved the accuracy of the kits filed under the Emergency Use Authorization.