The long-term health consequences of COVID-19
For a printable version of the February CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the definition and range of clinical symptoms associated with Long COVID
2. Describe the organ damage that occurs from COVID-19 and how this leads to ongoing medical issues
3. Describe the case definitions and symptoms associated with multisystem inflammatory syndrome (MIS)
4. Discuss hypotheses about why Long COVID occurs
After the initial onslaught, viral infections can be associated with long-term adverse consequences. This can result from direct damage from the primary infection, a persistent infection, or from downstream effects of the immune response to the initial infection. Disease occurring post-initial viral infection ranges in severity. At its most extreme, this can include some types of cancer, for example, following infection by Epstein-Barr virus, hepatitis B virus, hepatitis C virus, human immunodeficiency virus (HIV), and human papillomavirus (HPV).
On the other end of the disease severity scale, many infections are initially asymptomatic and self-limiting. This includes a mild clinical case of severe acute respiratory coronavirus 2 (SARS-CoV-2) that causes novel coronavirus disease 2019 (COVID-19). However, even when viral infections are asymptomatic, some people develop serious post-viral sequelae. Examples include infections associated with post-viral, developmental deficits following congenital viral infections, asthma following respiratory viral infections, immune suppression due to measles virus, and multisystem inflammatory syndrome (MIS) associated with SARS-CoV-2 infection.
We remain in a worldwide pandemic today with more than 400 million confirmed COVID-19 cases and more than five million deaths; consequently, interest is high in understanding the long-term effects of COVID-19. The attention given to these long-term COVID-19 sequelae is personal for many people. Others feel the impact on their communities and healthcare resources. Long-term COVID-19 also can be a disability: as of July 2021, Long COVID can be considered a disability under the Americans with Disabilities Act (ADA).
The term “Long COVID” seems to have stuck as the primary designation of extended symptomatology post primary infection with SARS-CoV-2. “#LongCovid” was first used by Elisa Perego, from Lombardy, Italy.1 This condition has also been referred to as post-COVID, long-haul COVID, post-acute COVID-19, long-term effects of COVID, or chronic COVID. The more technical term for this condition is “post-acute sequelae of SARS-CoV-2 infection” or “PASC.”
Long COVID complications
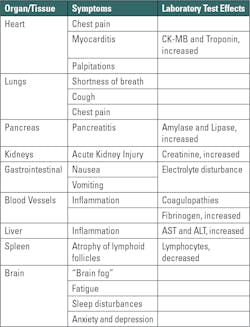
Long COVID complications may arise in a wide variety of organ systems including pulmonary, cardiovascular, hematological, pancreatic, dermatological, neurological, and psychological, to list just a few. (For expanded information on this topic there are excellent reviews and resources available.2,3,4) This persistence of symptoms — or the development of new symptoms — over many weeks and months post SARS-CoV-2 infection is consistent with a slow recovery and is generally considered to be Long COVID. Thus, Long COVID is defined as having a wide range of new, returning, ongoing health problems that are experienced a minimum of four weeks after initial COVID-19 infection, including initially asymptomatic people (Table 1).5,6,7
Unfortunately, the COVID-19 pandemic has also created and heightened social isolation that leads more people to have anxiety, depression, and mood changes — although it is important to recognize that these ailments do occur in the absence of a pandemic. Thus, connecting new, returning, or ongoing health problems with a prior COVID-19 episode is imprecise, especially at the individual patient level. For example, when evaluating the reasons for another common clinical event, a heart attack occurring while watching a sporting event, the cause could be related or unrelated to the excitement of watching the sporting event itself. In this instance, the underlying heart disease may have been a “ticking time bomb” that exploded during the sporting event. Accordingly, relating new symptoms to Long COVID-19 can be challenging, given the underlying health risks and conditions of any individual person.
Researchers at University of Michigan, led by Jeffrey Myers, MD, Professor of Diagnostic Pathology, examined chest X-rays and lung biopsies from patients who had COVID-19 and persistent respiratory symptoms. They found evidence of preexisting chronic lung disease in many patients, suggesting that COVID-19 exacerbated underlying lung disease that likely contributed to the development of Long COVID respiratory symptoms.9
Thus, for some people with Long COVID, the role of pre-existing, diagnosed or undiagnosed, underlying health issues (like the ticking time bomb predating a heart attack in the example above) may be an important contributory factor. Additionally, some studies show minimal differences between the prevalence of Long COVID symptoms between hospitalized and non-hospitalized COVID-19 patients, suggesting that, in some cases, the severity of COVID-19 is not the driving force behind the subsequent development Long COVID.10
An online Long COVID support group, Survivor Corps, solicited input from 5,163 group members. Participants listed, on average, 21 symptoms. The most common symptoms were fatigue (79%), headache (55%), shortness of breath (55%), difficulty concentrating (54%), cough (49%), changed sense of taste (45%), diarrhea (44%), and muscle or body aches (44%). The timing of symptom onset varied among respondents and was best described as happening in waves.11
Researchers at Mass General Brigham extracted 328,879 clinical notes from 26,117 COVID-19 positive patients in their post-acute infection period (days 51–110).12 Of 355 recorded symptoms, the most common were pain (43%), anxiety (26%), depression (24%), fatigue (23%), joint pain (21%), shortness of breath (21%), headache (20%), nausea and/or vomiting (20%), myalgia (19%), and gastroesophageal reflux (19%). Differences among studies reflect differences in the population studied, methodology, and SARS-CoV-2 variants.
An important feature of SARS-CoV-2 infection is that the virus doesn’t infect just the respiratory tract (nasal passage, throat, bronchus, and lungs). SARS-CoV-2 can also damage many other organs, including the heart, pancreas, kidneys, and brain. This is because the cell receptor for SARS-CoV-2’s entry is the angiotensin-converting enzyme 2 (ACE2) that is expressed on most types of cells throughout the body.
One of the most serious of the Long COVID effects is multisystem inflammatory syndrome (MIS), described by Bruno Larida in the January edition of Medical Laboratory Observer.14 Fortunately, to date, MIS is rare and only occasionally lethal. This medical condition is associated with inflammation in multiple body organs including the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal tract. In affected children, the peak of MIS is approximately one month after onset of COVID-19, with more than 6,400 children reportedly affected and among these, 55 deaths, according to the Centers for Disease Control and Prevention (CDC). Figure 1 provides a case definition of multisystem inflammatory disease for children (MIS-C). Adult cases have been reported, as well, and a separate list of criteria apply.
Long COVID can develop after either a SARS-CoV-2 infection or after a breakthrough infection following vaccination. Of several such studies, the largest and most representative is a study of 1.2 million British adults who had received, at least, one vaccine dose between December 2020 and July 2021. The researchers found approximately 5% of those with breakthrough COVID-19 infections reported Long COVID symptoms, compared with 11% of unvaccinated control group who had COVID-19.18
On the other hand, some patients with Long COVID appear to improve after vaccination.19 A Survivors Corps survey found that 42% of people with Long COVID reported improvement after vaccination. Another patient advocacy group, LongCovidSOS, found 58% of COVID-19 patients reported improvement of symptoms after vaccination.20 A third study found that 22% of patients with Long COVID improved after vaccination, but 31% of them did not improve. 21
One theory is that vaccination stimulates the immune system to target remnant viral particles; another is that some patients with Long COVID have an autoimmune response and that vaccination induces certain types of cytokines that can positively impact these autoreactive cells.
Why does Long COVID occur?
The exact mechanism(s) responsible for Long COVID complications remain unidentified. Several theories have been suggested — and any combination of these, or perhaps as-yet unidentified hypotheses, could be responsible for the development of Long COVID.22 The theories include:
Direct organ damage due to the primary SARS-CoV-2 infection of cells. As the ACE2 receptor on host cells that allow for SARS-CoV-2 infection is found throughout the body — including respiratory goblet cells, other epithelial and endothelial cells, gastrointestinal epithelial cells, pancreatic beta cells, and renal podocytes — the possibility exists that direct organ injury may be responsible for a portion of Long COVID cases.
Persistent reservoirs of SARS-CoV-2. Some studies have found remnant viral RNA in tissues. One study described a SARS-CoV-2 CD8 T-cell response of increased magnitude and breadth that was associated with prolonged SARS-CoV-2 PCR-positivity that extended over three months following the patient’s initial infection.23
“Viral ghosts” or viral remnants that persist in vivo. Improvement in symptomology in some Long COVID patients, subsequent to SARS-CoV-2 vaccination, provides some indirect evidence of the possible presence of such viral remnant material, which upon host immune stimulation by a vaccine, might help immune cells remove this residual viral material.
Prolonged autoimmune response to SARS-CoV-2 infection. The improvement in Long COVID symptomatology in some patients, subsequent to SARS-CoV-2 vaccination, provides some evidence that suggests there is a redirection of the host’s immune response, away from self and toward the virus, thus diverting the damaging autoimmune response.
Reactivation of other quiescent viruses. One study found Long COVID patients were more likely to have supplemental reactivation of the Epstein-Barr virus, the virus associated with mononucleosis. This finding suggests that SARS-CoV-2 caused reactivation of the Epstein-Barr virus in approximately 3 out of 4 patients with Long COVID, and this may be responsible for at least a portion of Long COVID cases.24
Unfortunately, there currently is no definitive diagnostic test for Long COVID. Further, some people with Long COVID have no direct laboratory evidence of prior SARS-CoV-2 infection. There are several reasons for a lack of documentation of a prior COVID-19 infection in patients with Long COVID. Those reasons, theoretically, include asymptomatic disease presentation; a lack of SARS-CoV-2 testing at the time of initial infection; use of SARS-CoV-2 “at home” rapid testing, for which there are no medical records of those results; or negative SARS-CoV-2 test results. One example of a negative test result that could occur during acute infection is SARS-CoV-2 serology (antibody testing). SARS-CoV-2 serology testing may provide a positive result in approximately 90% of people who were infected; therefore, approximately 10% will not have detectable antibodies (nucleocapsid protein), although they may have other evidence of recent infection (e.g., a positive NAAT assay result). Also, since the SARS-CoV-2 antibody response diminishes over time, the timing of the specimen collection could play a role in these cases.25
Diagnosis and treatment
Although there are no specific laboratory tests to diagnose Long COVID, testing may still be valuable in assessing symptoms or to point to other causes of symptoms that overlap those of Long COVID. Ruling in or out some other non-COVID-19 causes of a patient’s symptoms may be clinically and psychological helpful for an individual patient. For example, headaches and digestive symptomatology have many causes, and their appearance could have been triggered by COVID-19 or be completely unrelated. Thus, patients and their healthcare providers are encouraged, through shared decision-making, to adopt an approach that focuses on appropriate evaluation and goals of improving physical, mental health, and social wellbeing in those suspected of a Long COVID presentation. Because other medical conditions can develop independent of Long COVID, assigning symptoms to Long COVID may mask or delay evaluation of these other medical conditions.
Some medical centers have established Long COVID-19 clinics that specialize in the management of patients with these symptoms. Many social support groups are available online that connect people and provide outstanding assistance. Caution is needed, however, when considering such support avenues because social media has also spurred many support groups that may spread misinformation/disinformation.
Future direction
With more than 65 million confirmed COVID-19 cases (and many million more not confirmed or undiagnosed) in the United States, the shadow of Long COVID will be lengthy, adversely impacting the healthcare of millions of people and healthcare resources needed to serve them. Primary care physicians and specialists need training in Long COVID care. Clinics and other resources need to be quickly developed with wide accessibility.26 Clinical laboratories will provide a wide spectrum of tests to support the healthcare management of patients with Long COVID.
Currently, we have a poor understanding of the pathogenesis underlying Long COVID. Both basic and clinical research are required to understand risk factors, diagnosis, mitigation (beyond reducing risk of initial infection), and treatment, and management. Fortunately, the National Institutes of Health (NIH) recognizes this challenge. “Most likely it’s more than just one condition,” said Francis Collins, MD, Director of NIH, which is allocating $470 million for national studies on Long COVID. “The really troubling aspects of this terrible pandemic might be the lingering of this long-tail effect on people,” Collins said.27 With this strong support, we can expect closing many of the gaps in our understanding of Long COVID.
Harvey W. Kaufman, MD, is a board-certified pathologist, is Senior Medical Director at Quest Diagnostics. A 29-year veteran of Quest Diagnostics, Kaufman was an original and still active member of the Laboratory Working Group of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (formerly the National Kidney Disease Education Program).
William A Meyer, III, PhD, is Director, Medical Science Liaison, Infectious Diseases and Immunology at Quest Diagnostics where he has served for 40 years. In addition, he has collaborated with the National Institutes of Health and the Centers for Disease Control and Prevention on projects related to HIV, hepatitis, and COVID-19.
References:
William A Meyer, III, PhD, Director, Medical Science Liaison, Infectious Diseases and Immunology at Quest Diagnostics where he has served for 40 years. In addition, he has collaborated with the National Institutes of Health and the Centers for Disease Control and Prevention on projects related to HIV, hepatitis, and COVID-19.
Harvey W. Kaufman, MD, board-certified pathologist, Senior Medical Director at Quest Diagnostics. A 29-year veteran of Quest Diagnostics, Kaufman was an original and still active member of the Laboratory Working Group of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (formerly the National Kidney Disease Education Program).
About the Author

Harvey W. Kaufman, MD
a board-certified pathologist, is Senior Medical Director at Quest Diagnostics. A 29-year veteran of Quest Diagnostics, Kaufman was an original and still active member of the Laboratory Working Group of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (formerly the National Kidney Disease Education Program).

William A Meyer, III, PhD
is Director, Medical Science Liaison, Infectious Diseases and Immunology at Quest Diagnostics where he has served for 40 years. In addition, he has collaborated with the National Institutes of Health and the Centers for Disease Control and Prevention on projects related to HIV, hepatitis, and COVID-19.