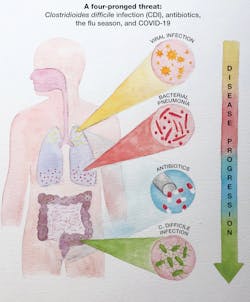
Managing the four-prong threat of CDI, antibiotics, the flu season and COVID-19
Antibiotic use increases during winter months because of the diagnosis or threat of secondary bacterial pneumonias following primary viral respiratory infections. Viral respiratory infections weaken the host’s immune and innate responses, allowing bacterial pathogens, such as Streptococcus pneumoniae and Staphylococcus aureus, to infect and inflame the lung alveoli. Physicians may implement antibiotic therapy after diagnosing bacterial pneumonia, but also often use antibiotics empirically as a preemptive strike when they suspect bacterial pneumonia.
The broad-spectrum antibiotics used to treat bacterial pneumonias are highly efficacious against lung bacterial pathogens. Unfortunately, they also are highly active against healthy intestinal microbiota. As a result, treatment for bacterial pneumonia results in a precipitous and unavoidable — at least until more specific antibiotics become available — drop in the diversity of the intestinal microflora. The consequence of this action may be devastating to patients because it compromises their defense against Clostridioides difficile infection (CDI). A patient treated with antibiotics for secondary pneumonia triggered by a viral respiratory infection now may face a third infection, a potentially life-threatening intestinal infection caused by a very dangerous toxin-producing bacterium.
The overuse of antibiotics in patients who have viral pneumonia hinders the efforts of antibiotic stewardship designed to minimize evolutionary stress that leads to antibiotic resistance. According to the Centers for Disease Control and Prevention (CDC), almost a third of S. pneumoniae isolates are resistant to one or more antibiotics, and methicillin-resistant S. aureus continues to pose a serious risk, especially to elderly patients. C. difficile ribotype 027, a hypervirulent strain that grows rapidly and produces higher levels of toxins A and B in the intestine, spread swiftly in the early 2000s because of its resistance to fluoroquinolone antibiotics. It continues to cause outbreaks in hospitals and healthcare facilities in Europe, Canada, and the U.S.
Antibiotics are prescribed more during a typical flu season, resulting in an increase in CDI
One of the first studies to examine the association of CDI with flu came from data collected over a 7-year period (1998-2005) in the Nationwide Inpatient Sample. Influenza activity preceded increased CDI activity both nationally and regionally.1 A 23% increase in CDI over summer months was observed, leading to the key observation that linked the increased use of antibiotics during flu season with increased rates of CDI. In another study, the seasonality of CDI was linked not only with influenza, but also with respiratory syncytial virus (RSV), another RNA virus associated with outbreaks in the winter.2 RSV is typically associated with respiratory infections in infants and young children, but it affects people of all ages. The increased rates of CDI were again associated with increased use of antibiotics; in this instance, primarily fluoroquinolones and macrolides that were administered to patients to lower the risk of bacterial pneumonia.
In one of the larger studies performed to date using U.S. National Hospital Discharge Survey data,3 pneumonia and influenza peak prevalence preceded peak CDI incidence by about 9 weeks. Importantly, there were intestinal effects from the CDI that, in some cases, lasted up to a year. Pneumonia and influenza peaks occurred earlier in some regions of the country, with similar early patterns being observed for increased rates of CDI. In all of these studies, the association of CDI seasonality was linked with increased use of antibiotics during the flu season. Other factors, such as hospital crowding and interhospital transfer, were considered, but increased antibiotic use was determined to be the primary triggering event.
The incidence of flu so far in 2021 has been historically low, and the 2020-2021 flu season has been anything but typical. Last year at this time, there were predictions of a very severe flu season for 2020-2021, in combination with the COVID-19 pandemic — a “twindemic” as some put it. However, the increased public health awareness and safety measures put in place for COVID — masks, social distancing, and reduced person-to-person contact — have led to a very low incidence of influenza infections. As countries that have an earlier flu season reported strikingly low numbers of cases, forecasts of a bad flu season in the U.S. turned instead to accurate predictions of a mild season. As of Spring 2021, only thousands of flu cases have been reported, compared to the millions reported in the previous year. The mitigation procedures used for COVID-19 have not only reduced flu rates, but the health and safety precautions used in healthcare facilities have helped to lower hospital-acquired infections such as CDI, even when the rate of antibiotic use has not been reduced.4
Another reason for reduced flu rates? People have been more inclined to get their flu vaccinations in the 2020-21 season. Because of the low flu rates, and probably also in part because of the decrease in hospital admissions during the 2020-2021 season, CDI rates that typically rise at the tail end of flu season likely will be steady or perhaps slightly lower this year. This is fortunate, but even so, CDI continues to be the predominant hospital-acquired infection in the United States, and C. difficile will continue to cause diarrhea and colitis in significant numbers of hospitalized patients and cause community-acquired disease.
COVID-19 infections early in the pandemic led to an overuse of antibiotics
In the early months of the pandemic when COVID-19 cases began to increase dramatically in the U.S. and in other countries, physicians started treating large numbers of patients with antibiotics in an effort to circumvent what they thought would be high numbers of bacterial pneumonia. In many locations in the U.S., more than 50% of COVID-19 patients were being treated with antibiotics. In some countries, the number approached 70%. The flu model of a close association of viral respiratory disease with complications caused by secondary bacterial pneumonia was the basis for this empirical approach early in the pandemic. Additionally, there were few, if any, other treatment options at this early stage in the pandemic. As this practice of empirical antibiotic therapy grew, infectious disease experts raised concerns about the appearance of antibiotic-resistant bacterial strains, along with possible consequences, such as CDI.5,6
Fortunately, by mid-2020, it became apparent that secondary bacterial pneumonias were not being seen at rates that were initially predicted. In fact, the opposite was true — secondary bacterial pneumonias were only being seen in low numbers of COVID-19 patients. Additionally, experts did not identify and associate any particular bacterial pathogens with the small number of patients who did develop bacterial pneumonia. Recommendations followed shortly thereafter, advising physicians to curtail empiric treatment of COVID-19 patients with antibiotics.7
Currently, researchers have not found a clear association between increased CDI cases and the high number of COVID-19 patients who received antibiotics in the early stages of the pandemic. The high use of antibiotics in the early months would suggest the possibility of a higher incidence of CDI. However, little data exist to confirm this hypothesis, or even to suggest that it was investigated, probably due to the overwhelming demands already placed on healthcare staffs, testing facilities, resources, and instrumentation.
SARS-CoV-2 may have multiple effects in the intestine
Recent clinical findings have revealed that up to 25% of COVID-19 patients develop diarrhea, and that large numbers of COVID-19 patients have detectable SARS-CoV-2 RNA in their feces.8 Perhaps this observation is not unexpected, since other respiratory viruses are known to trigger diarrhea in some patients. These findings also suggest the possibility of fecal-oral transmission of SARS-CoV-2. The ACE2 receptor for the virus exists in brush border membranes in the small intestine and in colonic crypts. The ACE2 receptor may, in fact, be present in higher amounts in the intestine than in the lungs. The binding of the virus to its receptor in the intestine is a plausible mechanism for COVID-19-associated diarrhea.
The ACE2 receptor may increase in amounts as we age, which seems contradictory to observations showing that COVID 19-associated diarrhea is more prevalent in younger adults.9,10 However, most of what is known about the binding of SARS-CoV-2 in the intestine is preliminary, and much remains to be discovered about the binding and action of SARS-CoV-2 in the intestine.
Although the binding of SARS-CoV-2 to its intestinal receptor probably represents the triggering event in COVID 19-associated diarrhea, physicians are now identifying COVID-19 patients who are co-infected with toxigenic C. difficile. In one study that looked at critically ill COVID-19 patients who received antibiotics and who were infected with C. difficile, 9 of 49 patients developed CDI.11 Other studies have confirmed co-infections with SARS-CoV-2 and C. difficile, and have shown that co-infected patients have more severe prognoses.12,13 The data at this time are slim, but more studies will provide additional information on the clinical relevance of C. difficile in COVID-19 patients.
In addition to the binding of SARS-CoV-2 to its intestinal receptor, the virus may have a negative impact on the healthy protective commensal bacteria in COVID-19 patients. This possibility has only recently been reported, but it suggests that SARS-CoV-2 infections may reduce the diversity of the microbiota.14,15 This finding is worth noting, because the disturbance of the microbiota is a predisposing factor for CDI. Other diseases and chronic conditions (e.g., norovirus, inflammatory bowel disease) that lead to a reduction in microbiota diversity have been associated with CDI.
We are at the infancy of our understanding of the SARS-CoV-2 mode of action and epidemiology and have much to learn about CDI. However, some early indications of the ability of some respiratory viruses to bind to intestinal receptors, cause diarrhea, and disturb our protective microbiota, which may serve as a triggering event for CDI, lead to important questions about the role of SARS-Co-V-2 infection in C. difficile epidemiology.
References
- Polgreen P, Yang M, Bohnett L, et al. A time-series analysis of Clostridium difficile and its seasonal association with influenza. Infect Control Hosp Epidemiol. 2010;31:382-387. doi:10.1086/651095.
- Gilca R, Fortin E, Frenette C, et al. Seasonal variations in Clostridium difficile infections are associated with influenza and respiratory syncytial virus activity independently of antibiotic prescriptions: a time series analysis in Quebec, Canada. Antimicrob Agents Chemother. 2012;56:639-646. doi:10.1128/AAC.05411-11.
- Brown K, Daneman N, Arora P, et al. The co-seasonality of pneumonia and influenza with Clostridium difficile infection in the United States, 1993-2008. Amer J Epidemiol. 2013:178:118-125. doi:10.1093/aje/kws463.
- Ponce-Alonso M, de la Fuente J, Rincon-Carlavilla A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection. Infect Cont & Hosp Epidemiol. 2020;1-5. doi:10.1017/ice.2020.454.
- Ferreira E, Penna B, Yates E. Should we be worried about Clostridioides difficile during the SARS-CoV2 pandemic? Front Microbiol. 2020;11: 581343. doi:10.3389/fmicb.2020.581343.
- Spigaglia P. COVID-19 and Clostridioides difficile infection (CDI): possible implications for elderly patients. Anaerobe. 2020;64:102233. doi:10.1016/j.anaerobe.2020.102233.
- Sieswerda E, de Boer M, Bonten M, et al. Recommendations for antibacterial therapy in adults with COVID-19 – an evidence based guideline. Clin Microbiol Infect. 2020;27:61-66. doi:10.1016/j.cmi.2020.09.041.
- Guo M, Tao W, Flavell R, et al. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev/Gastroenterol & Hepatol. 2020. doi:10.1038/s41575-021-00416-6.
- Vuille-dit-Bille R, Liechty K, Verrey F, et al. SARS-CoV-2 receptor ACE2 gene expression in small intestine correlates with age. Amino Acids. 2020;52(6-7):1063-1065. doi:10.1007/s00726-020-02870-z.
- Xu J, Chu M, Zhong F, et al. Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs. Cell Death Discov. 2020;6:76. Published 2020 Aug 11. doi:10.1038/s41420-020-00307-w.
- Sandhu, Tillotson G, Polistico J, et al. Clostridioides difficile in COVID-19 patients, Detroit, Michigan, USA, March-April 2020. Emerg Infect Dis. 2020;26:2272-2274. doi:10.3201/eid2609.202126.
- Lakkasani S, Chan K, Shaaban H. Clostridioides difficile in COVID-19 patients, Detroit, Michigan, USA, March-April 2020. Emerg Infect Dis. 2020;26:2299-2230. doi:10.3201/eid2609.202505.
- Granata G, Baroloni A, Codeluppi M, et al. The burden of Clostridioides difficile infection during the COVID-19 pandemic: a retrospective case-control study in Italian hospitals (CloVid). J Clin Med. 2020;9:1-11. doi:10.3390/jcm9123855.
- Zuo T, Zhang, F, Lui G, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944-955. doi:10.1053/j.gastro.2020.05.048.
- Gu S, Chen Y, Wu Z, et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020;71:2669-2678. doi:10.1093/cid/ciaa709.
About the Author

David M. Lyerly, PhD
is a Co-Founder and serves as Chief Science Officer for TECHLAB, Blacksburg, VA, which manufactures a variety of in vitro diagnostic tests for enteric diseases.

Jodie Y. Lee, MS, MBA
serves as Marketing Manager for TECHLAB. She has spent 16 years in the life science and diagnostic industries.