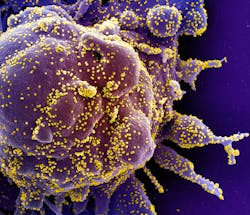
NIH organizes a public-private partnership for COVID-19 vaccine and treatments
The National Institutes of Health (NIH) and the Foundation for the NIH (FNIH) are bringing together more than a dozen leading biopharmaceutical companies, the Health and Human Services Office of the Assistant Secretary for Preparedness and Response, the Centers for Disease Control and Prevention, the Food and Drug Administration and the European Medicines Agency to develop an international strategy for a coordinated research response to the COVID-19 pandemic, NIH said in a press release.
Called the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), the partnership plans to develop a collaborative framework for prioritizing vaccine and drug candidates, streamlining clinical trials, coordinating regulatory processes and leveraging assets among all partners to rapidly respond to the COVID-19 and future pandemics.
Industry partners also will make available certain prioritized compounds, some of which have already cleared various phases of development, and associated data to support research related to COVID-19.
The partnership is being developed with input from a steering committee managed by the FNIH, which includes leaders from NIH, FDA and the research and development organizations of the companies.
The research community is currently striving to sift through more than 100 potential preventives and therapeutics for COVID-19, according to NIH.