Evaluating cardiac and renal risks in patients with diabetes: A “sweetheart” diagnostic challenge
For patients with diabetes, matters of the heart often carry a double meaning. While diabetes is associated with blood sugar, it is heart failure (HF) that increasingly threatens these “sweethearts.” Up to 22% of individuals with type 2 diabetes develop HF, even in the absence of coronary artery disease or hypertension.¹ When chronic kidney disease (CKD) is also present, a common comorbidity in this population, the risk rises significantly due to overlapping pathophysiology and cardiorenal interactions². Cardiac biomarkers, such as N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity troponin (hs-cTn), have demonstrated strong prognostic value in identifying patients at risk for HF before clinical symptoms emerge.² The intersection of diabetes, HF, and CKD highlights the need for earlier identification and intervention in asymptomatic patients.
Cardiac biomarkers overview: Troponins and natriuretic peptides
Cardiac biomarkers offer valuable insight into the subclinical processes of myocardial injury and stress that frequently accompany diabetes and CKD. Their clinical value now extends beyond acute diagnosis into long-term prognostication and chronic disease management.
High-sensitivity troponins
Cardiac-specific troponins reflect myocardial injury. High-sensitivity troponin assays can detect even very low concentrations of circulating troponin, indicative of subclinical cardiac strain. These “minimally elevated” levels, often present in stable patients, are independently associated with increased risk for future cardiovascular events and HF. Notably, troponin levels tend to be chronically elevated in CKD yet still retain prognostic significance. In the BiomarCaRE consortium, high-sensitivity cardiac troponin I (hs-cTnI) levels predicted both incident HF and cardiovascular mortality, with thresholds proposed to stratify risk across sexes.3
Natriuretic peptides (BNP/NT-proBNP)
BNP and NT-proBNP are secreted in equimolar amounts by ventricular myocytes in response to increased wall tension and volume overload.4 Despite this shared origin, they differ markedly in pharmacokinetics and clinical utility. BNP, the biologically active form, has a short plasma half-life of approximately 20 minutes and is cleared primarily by enzymatic degradation (e.g., neprilysin) and receptor-mediated pathways. In contrast, NT-proBNP is biologically inactive, has a longer circulating half-life of approximately 60 to 120 minutes, and is eliminated almost exclusively via renal excretion.4 These differences contribute to higher NT-proBNP plasma concentrations and greater analytical stability, making it especially useful in outpatient or chronic disease monitoring. While both biomarkers rise with declining renal function, studies have shown that they maintain independent prognostic value in patients with CKD.5
Analytical notes
High-sensitivity assays have enhanced the precision of cardiac biomarker measurement, enabling their use beyond acute care settings and into outpatient and preventive care. However, not all assays are interchangeable. For example, NT-proBNP and BNP differ in metabolism and clearance, with NT-proBNP having a longer half-life (60 to 120 minutes) compared to BNP (~20 minutes). Similarly, troponin I and T assays are distinct and require manufacturer-specific reference values. Interpretation is further refined by applying sex-specific and, in some cases, prognostic cutoffs to better personalize risk assessment.6
Biomarker roles in prognosis
Initially used to rule in or rule out acute myocardial infarction or HF, these biomarkers are now becoming recognized for their role in chronic disease risk stratification. Serial measurements allow clinicians to track disease trajectory and response to therapy, especially in asymptomatic high-risk groups, such as patients with diabetes and CKD. Their use in routine clinical decision-making is increasingly supported by professional society guidance.⁵
Prognostic value of biomarkers in diabetes and cardio-renal disease
High-sensitivity cardiac troponin assays provide powerful risk stratification in asymptomatic patients with diabetes. In community-based cohorts without known cardiovascular disease, each log-unit increase in hs-cTnI was associated with approximately a 1.4-fold higher risk of incident HF, independent of traditional risk factors.3 These associations support the use of sex-specific thresholds to refine risk prediction and identify individuals at high risk earlier in the disease course.
Similarly, natriuretic peptides (NPs) offer compelling prognostic value. In the U.S.-based Atherosclerosis Risk in Communities (ARIC) study, NT-proBNP levels were independently associated with increased risk of HF, cardiovascular events, and mortality in patients with type 2 diabetes, even at levels below conventional thresholds.7 Participants with NT-proBNP ≥125 pg/mL had an increased hazard for incident HF and cardiovascular death.
Using hs-cTn and NT-proBNP in combination further improves prognostic accuracy. Models incorporating both biomarkers have demonstrated excellent predictive performance, with patients exhibiting elevations in both facing significantly greater risk.7 The American Diabetes Association (ADA) now endorses the annual measurement of NT-proBNP or hs-cTn in asymptomatic diabetic patients to identify “Stage A/B” HF and enable early intervention.1
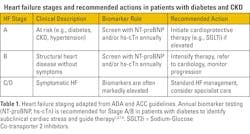
Heart failure stages and screening in diabetes
The 2024 ADA Standards of Care recommend measuring NP levels (BNP or NT-proBNP) annually in individuals with diabetes to identify those at increased risk of developing symptomatic HF.1,8 When elevated, these biomarkers warrant follow-up evaluation, such as echocardiography, to assess left ventricular hypertrophy, reduced ejection fraction, or diastolic dysfunction (See Table 1).
Supporting this strategy, both NPs and hs-cTn have demonstrated strong predictive value for identifying patients in Stages A or B who are likely to progress to symptomatic HF or cardiovascular death.1 Annual measurement of either biomarker enables clinicians to risk-stratify patients earlier and implement preventative therapies that may delay or avert progression.
Clinical case scenario: A 58-year-old man with type 2 diabetes, hypertension, and stage 3 CKD (eGFR 45) presents without HF (NYHA Class I). Labs show NT-proBNP at 150 pg/mL (normal <125) and hs-cTnI at 6 ng/L.
Interpretation: Elevated NT-proBNP suggests subclinical cardiac stress (Stage B HF), while detectable hs-cTnI reflects low-grade myocardial injury. Per ADA consensus, this asymptomatic patient is high-risk and should undergo further evaluation, such as echocardiography.¹
Action: Initiate or optimize cardioprotective therapy—start a sodium-glucose co-transporter 2 (SGLT2) inhibitor, optimize blood pressure (BP) control (e.g., ACEi/ARB), and encourage lifestyle changes. Monitor biomarkers over time to track progression or response.
Key point: Annual NT-proBNP or hs-cTn testing in asymptomatic diabetes can reclassify risk and guide early intervention.¹
Guideline-directed therapy and biomarker use: SGLT2 inhibitors and GLP-1 receptor agonists
SGLT2 inhibitors:
Large cardiovascular outcome trials—including EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58, DAPA-HF, and EMPEROR-Reduced—have consistently demonstrated that SGLT2 inhibitors significantly reduce the risk of HF hospitalization and cardiovascular (CV) death in patients including those with diabetes.9-13 Zinman and colleagues demonstrated consistent benefits across populations, with meta-analyses reporting a 25%–30% relative risk reduction in the composite of CV death or HF hospitalization with SGLT2 inhibitor therapy.9,10 These benefits apply to patients with and without reduced ejection fraction, as well as those with CKD. Notably, reductions in NT-proBNP and stabilization of troponin levels have been observed in clinical trials, supporting the use of biomarker-guided monitoring.13
GLP-1 receptor agonists:
GLP-1 receptor agonists, such as liraglutide, semaglutide, and dulaglutide, have shown efficacy in reducing major adverse cardiovascular events in patients with type 2 diabetes. Marso and colleagues demonstrated that these agents improve overall cardiovascular risk profiles in patients with established atherosclerotic cardiovascular disease (ASCVD).14 Their role in HF prevention is less consistent, but they remain a guideline-supported option. Elevated NT-proBNP or troponin levels may prompt clinicians to intensify therapy and prioritize SGLT2 inhibitors where indicated.
Implementation:
In clinical practice, high-risk patients with diabetes, particularly those in HF Stage A/B or with CKD, should be considered for evaluation for SGLT2 inhibitor therapy. The ADA consensus recommends prioritizing SGLT2 inhibitors in these patients when biomarkers indicate subclinical cardiac stress or injury.1,8
Conclusion
Cardiac biomarkers, such as hs-cTn and NPs, are no longer limited to acute care settings; they are now essential tools for managing chronic diseases. In patients with diabetes and CKD, these markers can unmask silent cardiovascular stress well before symptoms appear. Current guidelines recommend annual screening to enable timely intervention and personalized care.
By integrating prognostic biomarkers into routine clinical workflows, laboratory professionals and clinicians can transition from reactive to proactive care. This approach enables earlier risk reclassification, optimized therapy decisions, and the potential to reduce the long-term burden of HF in high-risk populations.
References
- Pop-Busui R, Januzzi JL, Bruemmer D, et. al. Heart failure: An underappreciated complication of diabetes. A consensus report of the American Diabetes Association. Diabetes Care. 2022;45(7):1670–1690. doi:10.2337/dci22-0014.
- Bansal N, Zelnick L, Go A, et al. Cardiac biomarkers and risk of incident heart failure in chronic kidney disease: The CRIC (Chronic Renal Insufficiency Cohort) study. J Am Heart Assoc. 2019;8(21):e012336. doi:10.1161/JAHA.119.012336.
- Yan I, Börschel CS, Neumann JT, et al. High-sensitivity cardiac troponin I levels and prediction of heart failure: Results from the BiomarCaRE Consortium. JACC Heart Fail. 2020;8(5):401-411. doi:10.1016/j.jchf.2019.12.008.
- Weber M, Mitrovic V, Hamm C. B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide - Diagnostic role in stable coronary artery disease. Exp Clin Cardiol. 2006;11(2):99-101.
- McCullough PA, Sandberg KR. B-type natriuretic peptide and renal disease. Heart Fail Rev. 2003;8(4):355-8. doi:10.1023/a:1026195332025.
- Apple FS, Wu AHB, Sandoval Y, et al. Sex-specific 99th percentile upper reference limits for high sensitivity cardiac troponin assays derived using a universal sample bank. Clin Chem. 2020;66(3):434-444. doi:10.1093/clinchem/hvz029.
- Gori M, Gupta DK, Claggett B, et al. Natriuretic peptide and high-sensitivity troponin for cardiovascular risk prediction in diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2016;39(5):677–685.
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/NEJMoa1504720.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi:10.1056/NEJMoa1812389.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. doi:10.1056/NEJMoa1611925.
- Inzucchi SE, Docherty KF, Køber L, et al; DAPA-HF Investigators and Committees. Dapagliflozin and the incidence of type 2 diabetes in patients with heart failure and reduced ejection fraction: An exploratory analysis from DAPA-HF. Diabetes Care. 2021;44(2):586–594. doi:10.2337/dc20-1675.
- Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: The EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140(21):1693–1702. doi:10.1161/CIRCULATIONAHA.119.042375.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi:10.1056/NEJMoa1603827.
About the Author

Jim Aguanno, PhD
received a Bachelor of Science in Chemistry and a Ph.D. in Biochemistry from Memphis State University. Following his Ph.D., he did fellowship in Laboratory Medicine at Washington University School of Medicine and Barnes Hospital in St. Louis, Missouri. Dr. Aguanno was Director of the Core Laboratory at Baylor University Medical Center in Dallas, Texas for 24 years. Dr. Aguanno joined Siemens Healthcare Diagnostics in January of 2004 and is currently Medical Science Partner in the Medical and Scientific Affairs group.

Justin Jones, PhD
has more than a decade of experience in diagnostics and translational science, including leading assay development projects at Siemens Healthineers. In his current position as a Field Medical Partner, he specializes in cardiac biomarkers and clinical education. Justin holds a PhD in biochemistry and is dedicated to connecting laboratory insights with patient care.