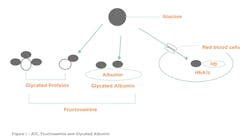
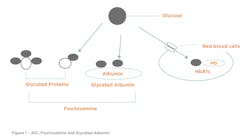
In blood, circulating proteins (hemoglobin, albumin, glycoproteins, immunoglobulins, and others) react with blood sugars (glucose) to form glycated protein products. Lab tests that measure the concentration of these glycated proteins are indicators of hyperglycemia and the level of glycemic control. These markers are also helpful in assessing the effectiveness of treatments and associated complications. Hemoglobin A1c (A1C) is the most widely used and understood of these markers and the most studied in terms of utility, risk, and outcomes.
As with any laboratory test, there are inherent advantages and limitations of the A1C test, and it is important to understand the situations where a test might be inaccurate or misleading due to a physiological condition or treatment. This article will review and summarize four important glycemic control tests that are employed for managing diabetes. Additionally, discussion will include glycemic location, lifespan, strengths, and weaknesses. We also describe a test called glycated albumin now available in the United States.
Diabetes
Diabetes mellitus is a chronic condition affecting millions of people around the world. Diabetes can lead to more serious complications depending on how long someone has had diabetes and how well it has been controlled. These complications include damage to the kidneys, eyes, limbs, and nerves. There is also a higher risk for developing coronary artery disease, stroke, and peripheral vascular disease. Diabetes is characterized by excessive thirst, frequent urination, weight loss, and decreased energy levels. According to the International Diabetes Federation (IDF), the estimated number of people with diabetes worldwide is around 537 million. Thus, approximately 1 in 10 of the world’s population have some form of the disease. In the United States, 32 million people or an age-adjusted prevalence of 10.7% of the American population are estimated to have diabetes.1,2
Diabetes is a chronic disease that occurs either when the pancreas does not produce enough insulin or when the body cannot effectively use the insulin it produces.2 Insulin produced by pancreatic cells (β-islet cells) regulate glucose levels in the blood. In healthy individuals, glucose levels are maintained within a narrow range by the physiological action of insulin. People with diabetes have damaged pancreatic cells (β-islet cells), so either they: 1) do not produce sufficient insulin (Type 1 diabetes), or 2) are insulin resistant (Type 2 diabetes).3,4 Since there is no cure for diabetes, effective treatment centers on managing clinically defined acceptable blood glucose levels.3,4
Type 1 diabetes is a condition characterized by high blood glucose levels caused by a lack of insulin and accounts for about 10% of all diabetics. People with Type 2 diabetes either lack insulin or their body is unable to use insulin efficiently (insulin resistance). Type 2 diabetes accounts for more than 90% of known disease.1,2 According to the American Diabetes Association (ADA) Standards of Medical Care in Diabetes 2021, the recommended goals for diabetics are to maintain blood glucose levels between 80–130 mg/dL and A1C levels below 7%.3 Therefore, management goals are to maintain glucose levels as close to normal levels as possible.3,4
A1C
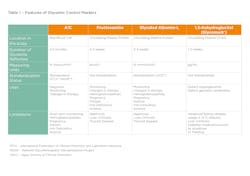
The gold standard test for glycemic monitoring is the A1C test (HbA1c) typically performed two–four times in a year. A1C is a fraction of hemoglobin that binds with circulating blood glucose in a process called glycation (Figure 1). If glucose elevates in the bloodstream, this in turn slowly elevates the A1C values. Therefore, A1C provides a retrospective view (2-to-3 months) of glycemic control and does not require fasting; for this reason, it is now used for both the diagnosis and monitoring of diabetes and associated complications.3,4 Table 1 provides more detail on the A1C test.
The ADA recommends that A1C tests utilized in the United States be certified by the National Glycohemoglobin Standardization Program (NGSP), thus providing a higher assurance of quality, comparability, and accuracy.3 The purpose of the NGSP is “to standardize hemoglobin A1C test results to those of the Diabetes Control and Complications Trial (DCCT) and United Kingdom Prospective Diabetes Study (UKPDS), which established the direct relationships between A1C levels and outcome risks in patients with diabetes.” 5,6,7
However, what happens if a patient with diabetes has less than or higher than normal red blood cells (RBCs), has a high RBC turnover rate, or has a genetic variant of hemoglobin? The A1C test result could potentially be inaccurate or misleading. There are physiological conditions and treatments that may affect the number and lifespan of the RBC in a patient, thus potentially providing an inaccurate A1C measurement.3,8,9 Table 1 describes these conditions in the “Limitations” section.
Fructosamine
There are alternative glycemic control tests that are not dependent on the level or lifespan of the red blood cell because they circulate freely in the bloodstream. For decades, an alternative test called fructosamine has been employed in these instances. The fructosamine test is a collection of different serum proteins that bind nonenzymatically to glucose to form stable ketoamines.10
The value of fructosamine tests is that proteins that undergo glycation are extracellular, in contrast to A1C, which is intracellular (Figure 1). Fructosamine is not affected by hemoglobin level, or red blood cell characteristics to which A1C is susceptible. This includes conditions such as hemoglobinopathies, sickle cell anemia, and anemia related to iron, vitamin B12, or folate deficiency.11 There are numerous methods for fructosamine determination, but the two common chemistry tests utilize either nitroblue tetrazolium (NBT) colorimetric procedure or an enzymatic spectrophotometric method. Fructosamine tests respond to changes in blood glucose more rapidly than A1C, within 2-3 weeks, which classify these assays as an “intermediate-term” glycemic control marker. Refer to Table 1.
Fructosamine assays primarily measure a circulating protein known as glycated albumin (GA). In the body, albumin is the most abundant circulating serum protein. When albumin undergoes glycation (binding with glucose) it is called glycated albumin. The fructosamine tests measure glycated albumin along with other glycated proteins such as glycated glycoproteins and glycated immunoglobulins. Fructosamine is consequently not specific for any one glycated protein in the serum; it measures different glycated proteins circulating in the bloodstream, which are all present at differing concentrations, proportions, and half-life.11,12
Glycated albumin (GA) test
In 2002, a Japanese company named Asahi-Kasei Pharma Corporation developed an enzymatic test specific for glycated albumin (GA). The inventor wanted a method that was not only specific for GA, but traceable to a known reference method. Additionally, the method for GA was enzymatic in architecture. There is peer-reviewed literature supporting the use of GA as a good marker of glycemic control from studies conducted in Japan, China, and the United States.13,14 In 2017, the product was cleared by the U.S. Food and Drug Administration (FDA) and commercialized in the United States in 2019. The GA is a user-defined enzymatic method for use on chemistry analyzers with open channel capability.
Because 60–70% of serum protein is albumin, fructosamine and GA share similarities in that both are “intermediate-term” glycemic control markers and not affected by hemoglobin level or red blood cell characteristics. Because the half-life of albumin is around 17 days, GA reflects blood glucose over a 2-to-3-week period. However, there are important distinctions between the tests for fructosamine and GA, most notably with specificity, reporting units and standardization. The GA test performs two separate reactions with the serum sample. This assay includes separate measurements of total albumin (bromocresol purple) and glycated albumin (enzymatic method). GA results are a ratio of glycated albumin to the total albumin — the GA reporting units (mmol/mol) differ from fructosamine reporting units (µmol/L).13,14
Another differentiator with the GA assay from Japan is that it is standardized to a known reference material provided by the JSCC (Japan Society of Clinical Chemistry) and is traceable to the gold standard reference for GA – isotope dilution liquid chromatography/tandem mass spectrometry.13,14
Detailed information for fructosamine and GA are described in Table 1.
1,5-Anhydroglucitol (1,5-AG)
1,5-Anhydroglucitol (1,5-AG) is a monosaccharide found in foods. It is a non-fasting blood test that reveals elevated postprandial glycemia (glucose “spikes” after meals) and/or glycemic variability. Patients with glucose levels within the normal range and <180 mg/dL have elevated levels of 1,5-AG (>10.0 ug/mL). Patients presenting with postprandial glycemia and/or glycemic variability have low levels of 1,5-AG (< 10.0 ug/mL).GlycoMark was the first 1,5-AG test on the market. This assay provides an estimate of the patient’s post-meal glucose over a 1–2-week period. The GlycoMark test can monitor the effectiveness of treatments that reduce postprandial glycemia and is a useful complement to the A1C test. GlycoMark is simple to perform as a user-defined method and it is well-evidenced. The 1,5-AG is a test that is complementary to A1C (used in conjunction with) to help assess recent postprandial glycemia and/or glycemic variability.15 Additional information for 1,5-AG is found in Table 1.
Conclusion
The A1C test is the gold standard for assessing glycemic control, it reflects mean blood glucose over a 2-to-3-month period and has strong predictive value for diabetes complications. A1C has limitations in which glycemic control is not accurately reflected in patients with conditions or treatments that affect red blood cells. Alternate glycemic control tests such as fructosamine or glycated albumin may be employed in patients with conditions or treatments that affect red blood cell turnover.
Fructosamine and glycated albumin are intermediate-term glycemic control markers that reflect mean blood glucose over a two to three-week period, and they are more sensitive to changes in glucose levels than A1C. The GA test has distinct differences from fructosamine. The GA test measures glycated albumin specifically and reports results as the ratio of glycated albumin in albumin. Also, the GA test is standardized to a reference method.
Lastly, 1,5-Anhydroglycitol is a marker that has been useful in assessing recent hyperglycemia and post-meal glucose spikes in patients with diabetes and can be used in a complementary manner with A1C. 1,5-AG is also used to monitor responses to treatments that assess recent postprandial glycemia and/or glycemic variability.15
References
- International Diabetes Federation. IDF Diabetes Atlas. Brussels Belgium: International Diabetes Federation. Accessed August 2, 2022. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html.
- Center for Disease Control and Prevention (CDC) Statistics and Facts page. Center for Disease Control and Prevention. Atlanta, GA. Accessed August 2, 2022. https://www.cdc.gov/datastatistics/index.html.
- American Diabetes Association. Standards of Medical Care in Diabetes 2021. Diabetes Care 2021; 44: S1-S226. doi: https://doi.org/10.2337/dc21-Sint.
- Winter W. An Expert’s Quick Guide to Diabetes Mellitus 2015 AACC Press.
- The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New England Journal of Medicine 1993; 329(14). doi: 10.1056/NEJM199309303291401.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). The Lancet. 1998; Volume 352, Issue 9131, 854-865.
- National Glycohemoglobin Standardization Project. NETCORE Lab, University of Missouri, Columbia, MO.
- Sickle Cell Trait and Other Hemoglobinopathies and Diabetes: Important Information to Providers. NIH Publication No. 14-6287. National Institute of Diabetes, Digestive and Kidney Diseases. National Institutes of Health (NIH). Bethesda, MD.
- Sacks DB, Arnold M, Bakris G. et al. Executive Summary: Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care 2011; 34(6): e61–e99. doi: 10.2337/dc11-9998.
- Mosca A, Carenini A, Zoppi F. et al. Plasma Protein Glycation as Measured by Fructosamine Assay. Clin Chem 1987, 33/7, 1141-1146.
- Gounden V, Ngu M, Anastasopoulou C, et al. Fructosamine. Updated 11, 2021. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Jan 2022.
- Armbruster D. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 1987; 33/12, 2153-2163.
- Kohzuma T, Yamamoto T, Uematsu Y. et al. Basic Performance of an Enzymatic Method for Glycated Albumin and Reference Range Determination. J of Diabetes Sci Technol. 2011; 5: 1455-1462. doi: 10.1177/193229681100500619.
- Kohzuma K, Koga M, Shimizu I et al. A Proposed Glycemic Control Marker for the Future; Glycated Albumin. Journal of Laboratory and Precision Medicine. 2019; 4: 23.
- Dungan K, Buse J, Largay J et al. 1,5-Anhydroglucitol and Postprandial Hyperglycemia as Measured by Continuous Glucose Monitoring System in Moderately Controlled Patients With Diabetes. Diabetes Care. 29(6):1214-9. doi: 10.2337/dc06-1910.
About the Author

Shane O’Neill
has 35 years of experience in the clinical diagnostics industry. He is currently the Global Director of Scientific Affairs, EKF Diagnostics. He is a keen advocate of new technologies and chemistries that bring new insights into patient care, particularly those associated with diabetes such as glycated albumin and beta-hydroxybutyrate.