Using HbA1c testing for diabetes diagnosis and management
Diabetes mellitus (diabetes) is a disease characterized by dysregulated glucose metabolism resulting in high blood sugar (hyperglycemia). Diabetes is a growing problem worldwide. In 2017, approximately 425 million adults (ages 20—79 years) had some form of diabetes. By 2045, it is estimated that this number will grow to 629 million—an increase of 48 percent—if preventive actions are not taken.1 Diabetes presents a significant health and financial burden. In 2017, diabetic complications were responsible for 4 million adult deaths worldwide, and diabetes-related healthcare expenditures topped $726 billion (USD).1
Insulin receptors
Cells require glucose for energy; however glucose cannot diffuse through most cell membranes. Cellular glucose uptake is regulated by the interaction of the pancreatic hormone insulin with cellular insulin receptors.2 Insulin is released from pancreatic beta cells in response to a carbohydrate-rich meal.3 Upon binding to the insulin receptor, a long-signal cascade assembles transmembrane glucose channels to admit glucose.2 Insulin levels decrease as glucose is sequestered. This feedback loop, along with glucose storage by the liver in the form of glycogen, helps to maintain blood glucose within a fairly narrow range.3 Diabetes occurs when either insulin production or receptor function become impaired.
Pathophysiology of diabetes
There are three primary types of diabetes. Type 1 diabetes (T1D) is a chronic and incurable autoimmune disease that usually occurs in childhood or adolescence but can develop later due to injury or other pancreatic disease. In T1D, T cells gradually destroy insulin-producing beta cells, creating insulin deficiency. Insulin production eventually becomes so deficient that it cannot support appropriate glucose regulation necessary for normal cell functionality.1,4,5
Type 2 diabetes (T2D) accounts for up to 95 percent of all cases in developed countries.1,4 T2D is primarily a disease of poor diet and weight management, and can develop at any age.1 While it too is a chronic disease, a recent large scale study demonstrated that remission can be achieved through significant calorie restriction and behavior modification.6 In T2D, cells become insensitive (resistant) to insulin due to insulin receptor or long signal cascade component malfunction. Reduced glucose uptake results in hyperglycemia.2 Inflammatory cytokines released from excess lipocytes also impair the action of insulin on insulin receptors.7 As T2D progresses, increased insulin production in response to hyperglycemia triggers beta cells to release of chemokines that result in their self-destruction by signaling IL-1β release by infiltrating macrophages.8
Gestational diabetes (GD) is diabetes or hyperglycemia that develops in the second or third trimester of pregnancy due to insulin resistance caused by interference of placental hormones. It can also have its origins in unrecognized impaired glucose tolerance or T2D prior to conception. GD can resolve shortly after birth, although Kitzmiller et al. report that postpartum glucose abnormalities can persist in 26.4 to 48.9 percent of women.9 GD increases the mother’s lifetime risk of developing T2D to about 60 percent. Gestational diabetes carries risks for both the mother and the developing fetus and requires close monitoring.
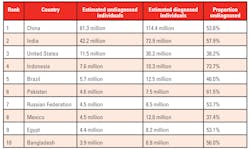
Impaired glucose tolerance (prediabetes) is diagnosed when blood sugar is consistently elevated above normal but remains below the T2D diagnostic cutoff. It may be present for several years before frank T2D is diagnosed. Individuals with impaired glucose tolerance are at increased risk of progressing to T2D. Fortunately, impaired glucose tolerance is reversible through lifestyle modifications, diet, and weight loss, however lack of awareness contributes significantly to the growing diabetes epidemic. (Table 1)1
Diabetes testing
Two primary tests are used to diagnose and monitor diabetes and prediabetes. Fasting or nonfasting blood glucose testing provides a moment-in time snapshot of glucose levels. Since glucose levels can fluctuate considerably over the course of hours and days, glucose testing cannot provide good insight into long term glycemic control. Hemoglobin A1c (HbA1c) measures a specific form of glycated hemoglobin and does not require fasting. Compared with fasting plasma glucose (FPG) and glucose tolerance testing, HbA1c is less affected by day-to-day variation in blood glucose levels: it reflects the average blood glucose level over the preceding 90 to 120 days, and thus provides more accurate information on glycemic control.4
Several major medical societies support the use of HbA1c. The American Diabetes Association recommends using HbA1c to diagnose diabetes and prediabetes. (Table 2)4 The Diabetes Canada Clinical Practice Guidelines recommend FPG or HbA1c screening of people over the age of 40 every three years.10 Because heart disease is a major cause of death and disability in diabetics, the European Society of Cardiology/European Association for the Study of Diabetes guideline recommends HbA1c and FPG for initial diabetes investigation, and the U.S. Preventive Services Task Force recommends similar screening as part of cardiovascular risk assessment in adults aged 40 to 70 years with specific risk factors.11,12
What is hemoglobin A1c?
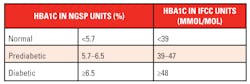
The most common type of hemoglobin is HbA, which is comprised of two αsubunits and two βsubunits. Unlike other cells, red blood cells (RBC) are permeable to glucose. Glucose covalently binds with the amino groups of valine and lysine residues of both subunits in a slow non-enzymatic reaction called glycation, but the N-terminal valine residues of hemoglobinββ chains are particularly susceptible.13 When hemoglobin is fractionated using HPLC, N-terminal glycated Hb elutes as the 4th peak after HbA0 (pure hemoglobin), so was designated HbA1c.14 The percentage of glycated hemoglobin relative to total hemoglobin increases as blood glucose levels increase.14,15 The U.S. National Glycohemoglobin Standardization Program (NGSP) recommends reporting this fraction as % HbA1c, however in recent years there has been a move toward standardization and reporting in HbA1cmmol/total Hbmol as recommended by the IFCC. (Table 2)15
Once glucose has bound covalently, it remains attached to hemoglobin until the blood cell containing it dies. The life span of human red blood cells is approximately 120 days (four months), however not all cells are formed or die at the same time. As a result, the quantity of glycated hemoglobin determined by an HbA1c test represents the average blood glucose levels over a two- to three-month period, however accurate determination can be affected by disorders that shorten RBC lifespan.14,16
HbA1c can be measured using different types of chromatography, immunoassay using antibodies directed against the N-terminal glycated valine, and enzymatic assays. Tests for HbA1c continue to migrate from HPLC to integrated lab instruments. In the enzymatic process, whole blood is hemolyzed and treated with an oxidizing agent to expose hemoglobin and convert it to MetHb. A protease is added which cleaves glycated MetHbA1c to yield metHbA and fructosyl dipeptide. Absorbance is measured at 478/805 nm to calculate the total hemoglobin concentration. In a second reaction, fructosyl peptide oxidase is added to the fructosyl dipeptide to produce hydrogen peroxide (H2O2), which reacts with a coloring agent in the presence of peroxidase (POD) to develop color. The change in absorbance measured at 658/805 nm is used to calculate the HbA1c concentration. (Figure 1)
Potential confounders of HbA1c assays
Mutations in genes encoding the α and β subunits result in changes to the hemoglobin protein. Over 1000 variants have been recorded. The majority of variants have no effect on the production, structure or function of hemoglobin, however others are associated with diseases. For example, the HbS variant is associated with sickle cell trait and sickle cell anemia while homozygosity for HbC results in mild hemolytic anemia.
The most common variants worldwide (in order of approximate prevalence) are HbS, HbE, HbC, and HbD. Even though each of these variants possess the β-chain terminal valine, they deviate from HbA by only one or two other β-chain residues. This can be enough to shift the expected HbA1c peak, affecting the performance of chromatographic assays based on net charge.13,17 Some immunoassay systems have also been reported to give falsely elevated results in the presence of variants.17 Enzymatic assays are less prone to interference by these variants.
HbF is the fetal form of hemoglobin. It possesses two γ subunits in place of the β subunit and lacks an N-terminal valine. The N-terminal residues (glycine in HbF type G and alanine in type A) are available for glycation in approximately 80-85 percent of HbF molecules. Only about 40 percent of total glycation actually occurs at these residues, however, and at approximately 25–33 percent the rate of β-chain glycation, thus a much lower percentage of HbF is glycated. HbF accounts for approximately two percent of hemoglobin in most individuals and does not interfere with HbA1c determination. The percentage of HbF can, however, be much higher in individuals with certain hemoglobinopathies, such sickle cell anemia and β thalassemia, because the persistence of fetal hemoglobin can help compensate for hemoglobin defects.
In addition, HbF can account for ≥30 percent of total hemoglobin in individuals with hereditary persistence of fetal hemoglobin not associated with disease. In each of these cases, elevated HbF can interfere with some chromatographic, immunoassay, and enzymatic assays. Patients and physicians are typically aware of their status before testing, although some individuals with elevated HbF are identified upon receiving unusual HbA1c results.16,17
In conclusion, HbA1c provides valuable information that can aid in the diagnosis and management of all types of diabetes.
REFERENCES
- International Diabetes Federation. IDF Diabetes Atlas, Eighth edition. 2017; https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html.
- Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6(1).
- Mann E, Bellin M. Secretion of Insulin in Response to Diet and Hormones. Pancreapedia: Exocrine Pancreas Knowledge Base. 2016.
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13-S28.
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. The Lancet. 2014;383(9911):69-82.
- Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. The Lancet. 2018;391(10120):541-551.
- Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1-4.
- Eguchi K, Nagai R. Islet inflammation in type 2 diabetes and physiology. J Clin Invest. 2017;127(1):14-23.
- Kitzmiller JL, Dang-Kilduff L, Taslimi MM. Gestational diabetes after delivery. Short-term management and long-term risks. Diabetes Care. 2007;30 Suppl 2:S225-235.
- Ekoe JM, Goldenberg R, Katz P, et al. Screening for Diabetes in Adults. Can J Diabetes. 2018;42 Suppl 1:S16-S19.
- Ryden L, Grant PJ, Anker SD, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD - summary. Diab Vasc Dis Res. 2014;11(3):133-173.
- United States Preventative Services Task Force. Final Recommendation Statement: Abnormal Blood Glucose and Type 2 Diabetes Mellitus:Screening.2018; https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes.
- Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009;3(3):446-451.
- Peterson KP, Pavlovich JG, Goldstein D, et al. What is hemoglobin A1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin Chem. 1998;44(9):1951-1958.
- Miedema K. Standardization of HbA1c and Optimal Range of Monitoring. Scand J Clin Lab Invest Suppl. 2005;240:61-72.
- Rhea JM, Koch D, Ritchie J, et al. Unintended reporting of misleading Hb A(1c) values when using assays incapable of detecting hemoglobin variants. Arch Pathol Lab Med. 2013;137(12):1788-1791.
- Little RR, Rohlfing CL, Hanson SE, et al. The effect of increased fetal hemoglobin on 7 common Hb A1c assay methods. Clin Chem. 2012;58(5):945-947.
About the Author

H. Roma Levy, MS
holds an MS from UC Santa Cruz in molecular biology with an emphasis in chronobiology, in which she conducted independent research. As a medical writer for Siemens Healthineers, Ms. Levy has written or co-authored multiple articles and clinical educational presentations over the last 16 years in diverse areas, including immunology and infectious disease, endocrinology, cardiology, and opioid addiction.