The impact of red blood cell lifespan on HbA1c measurement
Diabetes mellitus affects over 30 million Americans, and 1.5 million Americans are diagnosed with diabetes every year. In addition to monitoring whole blood glucose, it is recommended by the American Diabetes Association (ADA) to test diabetic patients for hemoglobin A1c (HbA1c) two to four times per year. The completed HbA1c results in a patient’s medical record is used as an indicator of the quality of medical care and can play a role in monetary reimbursement. Regarding these guidelines and reimbursement practices, and knowing the absence of a HbA1c data point may result in a lowered quality score for the clinician, are there clinical reasons why a patient should not have HbA1c reported or be reported with caution? This article will discuss the role of red blood cell (RBC) lifespan on HbA1c results, clinical interference on HbA1c results, and cases where HbA1c should not be reported for clinical reasons. This article will provide a perspective to those laboratorians who, depending upon the test method, must answer the question, “Why didn’t the laboratory provide a result on my patient today?”
Assumptions related to RBC survival
The HbA1c results are used to provide an estimation of the patient’s glycemic control over the last two to three months, assuming the RBCs have an average circulating lifespan of 120 days. During that time period, glucose in the blood permanently binds to the hemoglobin in the RBC by the Amadori rearrangement forming HbA1c from the wild type (or typical) HbA. The higher the level of circulating glucose the higher the percentage of HbA1c will be formed, in turn, an average estimated glucose level (eAG) can be calculated from the percentage of HbA1c. Recently Cohen, et al, has summarized altered RBC lifespan will affect the eAG from a calculated HbA1c result.1
Current interpretation of HbA1c values, which corresponds to the calculated (eAG), assumes that the RBC life span is the same for all patients. However, even modest variation in red cell survival—that would not be apparent in routine hematological studies—could have a significant impact on the HbA1c level.2 Therefore, the detection of some of the more common causes of decreased (or increased) RBC survival would be important in determining whether the HbA1c level was an accurate reflection of a patient’s level of glycemic control. In general, a shorter RBC life span would yield lower levels of HbA1c at a given average whole blood glucose concentration as compared to that of a normal patient.
Extrinsic causes of decreased RBC survival include pernicious anemia, acquired hemolytic anemia, pregnancy, nephritis, hepatic disease, burns, sepsis, and anemia associated with malignancy. Intrinsic causes include hemoglobinopathy, paroxysmal nocturnal hemoglobinuria, congenital hemolytic jaundice, and elliptocytosis. Renal and hepatic disease may be detected by scrutiny of the results of routine serum chemistry profiles. Hemolytic anemia is rare, and may be suspected with a normocytic, normochromic pattern of anemia. Rarely will a patient with diabetes have testing which is specifically focused on determining if red cell survival is diminished due to congenital causes, with the most common condition being the presence of a hemoglobinopathy.
Interferences to be considered
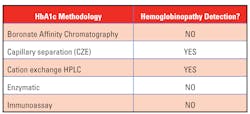
- Analytical interference: Most newer methods for HbA1c have minimal analytical interference from the presence of the major hemoglobin variants (HbS, HbC, HbE, HbD) in the specimen. The reader is referred to the NGSP (National Glycohemoglobin Standardization Program) website for a more detailed table by manufacturer and methodology.
- Clinical interference: There are clinical conditions which will limit the ability to use the HbA1c value as an estimate of the degree of glycemic control. “This issue is of particular concern when using assays for HbA1c (e.g. immunoassay) that will produce an HbA1c result for homozygous Hb variants, without providing information that an Hb variant is present in the sample.”3
The summary of the 2018 Standards of Medical Care in Diabetes by The American Diabetes Association stresses that the A1c test can give skewed results in people with certain genetic traits that alter the molecules in their red blood cells, such as hemoglobinopathies.3
Most methods are free from analytical interference from common hemoglobinopathies however, the clinical interference may not be known if the patient’s results do not indicate the presence of a hemoglobinopathy or other disease state that can alter the RBC lifespan.4
The decision related to the method to be used for measurement of HbA1c would be easier if one knew that each patient being tested had a normal RBC lifespan. If this were the case, then the decision could be made based on test cost and the ability to automate the pre and post-analytical components of this analysis. Unfortunately, there is a small percentage of patients being tested by non-separation methods, such as immunoassay, enzymatic, or boronate affinity, that have undetected shortening of RBC survival, to a degree that will cause a reduction in HbA1c that is unrelated to the patient’s average glucose level during the prior two to three months. How is one to determine when HbA1c, in the presence of a disease state, such as a hemoglobinopathy, is clinically inaccurate due to RBC survival issues? A methodology that indicates if a hemoglobin variant is present must be used.
It has been suggested that prior clinical information in the medical record could be reviewed to determine if there are any conditions that will cause significant shortening of the red cell survival time. This approach may not be feasible in a high-volume reference laboratory, or in facilities that have isolated medical records for outpatient and inpatient encounters. It will not be useful in selecting patients that have a clinically silent hemoglobinopathy but have never been tested for this condition.
Another solution is to implement a method for HbA1c testing which will also detect most hemoglobinopathies and allow the laboratory to report the comment: “The presence of a hemoglobinopathy in this patient may cause a reduction in red cell survival, which could falsely reduce the measured HbA1c level. Please consider fructosamine or glycated albumin testing to monitor this patient’s level of glycemic control.”
In many healthcare systems, the use of HbA1c is mandated by predetermined practice guidelines that are tied to reimbursement and a quality scorecard. These electronic monitoring systems cannot accept this comment as satisfying the requirement for a quantitative HbA1c result. It may be necessary to modify these quality systems to allow for these selected patients to meet the quality guidelines by alternative testing methods.
Summary
HbA1c testing has been promoted as a required test to monitor glycemic control in all diabetic patients. Many methods have been evaluated for analytical interferences from the presence of common, abnormal hemoglobin molecules, and laboratory acceptance of certain methods have been based solely on a manufacturer’s claim of lack of analytical interferences.
There is growing evidence that it is also important to identify the clinical status of a patient where there is significantly decreased red cell survival, as the HbA1c will be falsely lowered. While many conditions which shorten RBC life can be suspected by a review of the patient’s medical record or prior laboratory results, there are patients with inherited abnormalities in hemoglobin structure or globin synthesis rate that have reduced RBC survival times which are clinically silent. These are the patients that will benefit from the use of a method for HbA1c that highlights the presence of the abnormal hemoglobin molecules.
In these cases, one is not seeking a HbA1c result that is free of analytical interferences, but instead one which allows the testing to be directed to another method which is less dependent on the assumption of a normal RBC lifespan, such as fructosamine or glycated albumin.
REFERENCES
- Cohen R, Franco R, Khera P, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112(10):4284-4291.
- Sacks D. Measurement of Hemoglobin A1c: A new twist on the path to harmony. Diabetes Care. 2012;35(12):2674-2680.
- Rhea J, Koch D, Ritchie J, et al. Unintended Reporting of Misleading HbA1c Values When Using Assays Incapable of Detecting Hemoglobin Variants. Arch Pathol Lab Med. 2013;137(12):1788-1791.
- Summary of Revisions: Standards of Medical Care in Diabetes. 2018. Diabetes Care. 2017;41(Supplement 1): S4-S6.
About the Author

Thomas P. Lohmann, MD
serves as Director of Medical and Scientific Affairs for Sebia-USA. Lohmann is board certified in Anatomic and Clinical Pathology and has 40 years of experience in Laboratory Medicine.