Hemoglobin A1c testing and diabetes management
The Diabetes Research Institute Foundation has estimated a 50 percent increase in the number of people living with diabetes mellitus in the United States over the past decade.1 With more than 400 million people living with and managing diabetes worldwide, the ability to accurately diagnose and track patient management is a growing need. The diagnosis of diabetes mellitus uses a combination of measurements: fasting serum glucose levels, presentation of symptoms, two-hour plasma glucose levels during a glucose tolerance test, and hemoglobin A1c (HbA1c) levels.2 Current patient management includes diet, exercise, medication, daily monitoring of blood glucose, and HbA1c monitoring.
Mayo Clinic Laboratories emphasizes the value of controlling glucose levels to prevent long-term complications such as retinopathy, neuropathy, and cardiovascular disease. However, solely measuring and monitoring blood glucose levels has some limitations as the test only measures glucose levels at the time of testing and it relies on the patient to consistently test their levels at home, using a point-of-care device. To address these limitations and provide a broader indication of long-term glycemic control, HbA1c testing is used. It is typically performed in a laboratory setting and the test indicates the patient’s average levels of blood glucose over the past 8 to 12 weeks. The NGSP, originally called the National Glycohemoglobin Standardization Program, supports the American Diabetes Association’s recommendations that patients who are meeting glycemic goals be tested for HbA1c twice a year, while patients not meeting glycemic goals or patients with changes to therapies be tested every three months.2,3 The American Diabetes Association sets a normal patient at < 5.7 percent, prediabetes patients at ≥ 5.7-6.5 percent, and diabetic patients at ≥ 6.5 percent HbA1c.4
Hemoglobin is the protein in red blood cells that transports oxygen.5 HbA1c is a form of hemoglobin that is generated by a non-enzymatic glycation pathway, following a Schiff’s base reaction and an Amadori rearrangement that occurs between glucose and the N-terminal valine of the hemoglobin beta chain.2 This glycosylation is irreversible and occurs continually over the entire 8 to 12 week life span of a given erythrocyte.3,5 This binding reaction reflects the average level of glucose that young and old red blood cells are exposed to over the course of 8 to 12 weeks. A blood sample will have a population of young-to-old red blood cells and the percent of HbA1c that is measured is a result of an average glucose level in the patient’s sample over this time period. HbA1c is an excellent analyte to monitor a patient’s glycemic control or therapeutic intervention.
*Turbidimetric, chemiluminescent and agglutination methods are antibody based assays
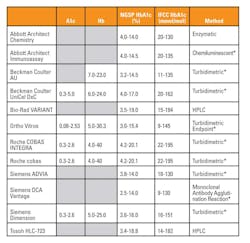
Methods for measuring HbA1c include high pressure liquid chromatography based (HPLC), antibody based (immunoassay), and enzyme based (enzymatic) methods. Table 1 lists, in alphabetical order, twelve of the most common HbA1c assays with their claimed reportable ranges and reaction methods. The advantages and disadvantages of each should be considered in order to choose a method that best fits the patient population for which the laboratory is reporting results.
With treatment standards being set by various governing associations across the world, it is critical that the values being reported to patients and professional caretakers be accurate. Prior to standardization, results were observed to vary ±4-8.1 percent on the same sample.5 The accuracy of commercially available HbA1c tests has been improved by the International Federation of Clinical Chemistry Working Group (IFCC-WG) on HbA1c.3 This group established reference materials and methods for HbA1c that include values assigned by mass spectrometry and capillary electrophoresis.3 A master equation was developed to demonstrate the relationship between NGSP measurements expressed as the %HbA1c of total hemoglobin and IFCC expressed in SI units (mmol/mol), where the relationship is: NGSP = (0.09148 * IFCC) + 2.152.3 The IFCC primary reference material uses a mixture of purified HbA1c and HbAo that were isolated using cation exchange and affinity chromatography and is considered the only valid standardization for HbA1c.3
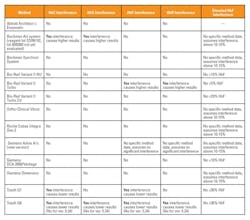
Accurately reporting HbA1c levels can be further complicated by patients with other clinical conditions or high levels of hemoglobin variants that interfere with HbA1c results. For example, patients with clinical conditions that lengthen the lifespan of their red blood cells (polycythemia) can produce falsely high HbA1c results, while patients with red blood cells with shorter life spans (hemolytic anemia) may produce HbA1c values lower than actual.2,5 Patients with homozygous or double heterozygous forms of abnormal hemoglobin (CC, SS, EE, SC) have no hemoglobin A present and therefore no HbA1c can be measured in these patients.2 Table 2 is a summary from the NGSP website of potential hemoglobin variants and their effect on common methods. The NGSP criteria used to determine whether or not a method shows interference that is clinically significant is > ±7 percent at 6 percent and/or > ±7 percent at 9 percent HbA1c.3 The table shows that HbC and HbD do not interfere with the majority of the methods, with the exception of the Beckman AU and Tosoh G8 and G7. The advantage to HPLC methods (e.g. Tosoh and BioRad systems) is that the variants that can affect results will be detected in the chromatogram analysis and samples can be retested if necessary.
Establishing, validating, and verifying the linear reportable range claims of these methods is another critical component to reporting accurate patient results. Calibration Verification and Linearity experiments are standard procedures of practice in order to fulfill CLIA ‘88, CAP, ISO 15189, COLA, JCAHO, and JCI testing requirements and occurs on a frequency of at least once every 6 months and ideally using materials which are commercially available.6,7 They are manufactured to predefined concentrations and have recovery targets optimized to the claimed reportable range for various instrument vendors (e.g. Roche cobas 6000 and Tosoh G8). It is important to select materials that are designed to the type of method. For example, materials used on the Tosoh G8 analyzer (an HPLC method) must preserve all forms of hemoglobin integrity, thus limiting aberrant chromatography peaks caused by hemoglobin alterations or degradation. Immunoassay methods must preserve the specific epitope recognized by the antibodies for HbA1c that the manufacturer employs for the method.
Alongside the increasing number of people living with and managing diabetes, the ability to accurately diagnose prediabetes and diabetes patients also becomes critical. Standardization of results across platforms, and worldwide by the IFCC-WG, has resulted in more accurate results and it now becomes the reagent manufacturer’s responsibility to continue to ensure their methods are traceable to the IFCC reference material and method. Laboratories have the continued responsibility to ensure the methods they are using to report HbA1c values are providing accurate results by running Internal Quality Controls (IQC), Calibration Verification and participating in Proficiency Testing, and External Quality Assurance (EQA) schemes.
The more recent trend toward point-of-care testing for HbA1c adds value to professional caretakers, providing visibility to the patient’s HbA1c trends and allowing for real-time adjustment to patient care plans. However, as this test moves out of the laboratory and toward point-of-care where regulations are different, considerations for the specificity, precision, accuracy, and reportable range of these methods needs to be a focus.5
REFERENCES
- What is Diabetes? Diabetes Research Institute Foundation website https://www.diabetesresearch.org/what-is-diabetes. Accessed November 26, 2018.
- Test ID: HbA1c. Mayo Clinic Laboratories website https://www.mayomedicallaboratories.com/testcatalog/Clinical+and+Interpretive/8208. Accessed November 26, 2018.
- Clinical Use, HbA1c Assay Interferences and IFCC Standardization. NGSP website http://www.ngsp.org/index.asp. Updated October 2018. Accessed November 26, 2018.
- Diagnosing Diabetes and Learning about Prediabetes. American Diabetes Association website http://www.diabetes.org/diabetes-basics/diagnosis/?loc=db-slabnav. Accessed November 26, 2018.
- Gupta S, Jain U, Chauhan N. Laboratory Diagnosis of HbA1c: A Review. Journal of Nanomedicine Research. 2017; 5(4):00120. DOI: 10.15406/jnmr.2017.05.00120.
- Linearity and Calibration Verification FAQs. LGC Maine Standards website https://www.mainestandards.com/support/faq-calibration-verification.php. Accessed November 27, 2018.
- CLIA ’88. 42 CFR 493 in section 493.1253. Code of Federal regulations website. https://www.gpo.gov/fdsys/pkg/CFR-2003-title42-vol3/xml/CFR-2003-title42-vol3-part493.xml#seqnum493.1253. Accessed November 27, 2018.
About the Author

Jessica Pawlak
holds a Bachelor of Science in Biochemistry from Boston College, and a Master of Science in Biochemistry from the University of Maine. Her career includes work in both academia and industry, as well as an internship at the National Institutes of Health. Jessica joined LGC Maine Standards in February 2010 as a Research Scientist I and is currently the R&D Manager overseeing LGC Maine Standards new product development and product support activities including the HbA1c product line.
Michael Sweatt
serves as General Manager, LGC Maine Standards
Ralph Ito
serves as Chief Scientific Officer, LGC Maine Standards
Catherine Cahill
serves as Technical Support Supervisor/Product Manager, LGC Maine Standards