Advancements in techniques and technologies in oncology
To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
- List the epidemiological statistics on current cancer rates and deaths in the United States.
- Define how cancer is diagnosed.
-
Discuss laboratory testing, artificial intelligence, endoscopic exams, molecular testing, and biopsies as they relate to cancer diagnosis and treatment.
- List new innovations in the evolution of cancer treatment.
According to the National Cancer Institute, oncology is a branch of medicine that specializes in the diagnosis and treatment of cancer.1 Cancer is a health challenge across the globe including in the United States. More than 2 million new cancer cases and over 600,000 deaths are projected to occur in the United States in 2025 due to cancer.2 Thanks to advancements in the techniques and technologies in oncology, cancer mortality rates have continued to decline through 2022 averting nearly 4.5 million deaths since 1991.2
Mechanism of cancer
The mechanism of cancer involves dysregulation of the cell cycle and the disruption of key checkpoint controls by manipulating and overcoming the normal regulatory processes that prevent uncontrolled proliferation.3 Cancer cells utilize different metabolic strategies to sustain rapid growth; accumulate multiple genetic changes; leave the tissue of origin and spread to other sites; evading the immune system, which typically eliminates abnormal or damaged cells; and increase the supply of nutrients and oxygen to tumors.4
Diagnosis of cancer
Cancer can cause various symptoms, many of which are similar to that of benign tumors or other causes.5 Hence, an accurate diagnosis of cancer involves knowledge of the patient’s history, physical examination, and diagnostic testing. Cancer diagnosis is usually confirmed by tests using various methods.
The various testing methods used for cancer diagnosis are as follows: 6
- Laboratory tests
- Molecular testing
- Diagnostic imaging
- Endoscopic exams
- Tumor biopsies
Diagnostic tests for cancer are used to confirm or eliminate the presence of cancer; monitor the disease progression; and evaluate the effectiveness of treatment. In some cases, it is necessary to repeat testing when a person's condition has changed, if a sample collected was not of good quality, or an abnormal test result needs to be confirmed.
Laboratory tests for cancer
Some of the common laboratory tests are as follows:
Complete blood count (CBC): Measures levels of red blood cells, white blood cells, and platelets. Abnormally high or low counts can indicate certain cancers, such as leukemia or lymphoma.
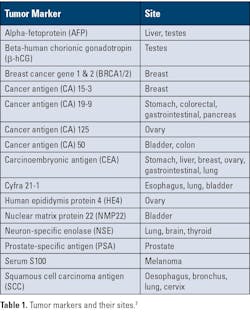
Tumor markers: Measure substances—often proteins—made by cancer cells or by the body in response to cancer. They are usually used with other tests to help detect, monitor, or track treatment response. (See Table 1 for tumor markers and their sites.)
Molecular testing: Molecular testing is a laboratory method that uses a sample of tissue, blood, or other body fluid to check for certain genes, proteins, or other biomolecules to examine genetic and molecular characteristics. The tests are performed to provide information for diagnosis, prognosis, and treatment planning. Molecular testing for cancer can be broadly classified into three types — DNA analysis, mRNA expression analysis, and protein expression analysis.8
DNA analysis
DNA analysis looks at the genetic material of cells to find changes (mutations, amplifications, and alterations) that may cause cancer to develop, grow, or spread. Depending on the type of analysis required, the following techniques may be used:
- Polymerase chain reaction (PCR)
- Real-time PCR
- Microarray
- Sequencing – Sanger sequencing and next-generation sequencing (NGS)
Examples of point mutations are the following:
- BRAF V600E point mutation in the BRAF gene - in melanoma - the amino acid valine (V) at position 600 is replaced by glutamic acid (E).9
- EGFR L858R point mutation in the EGFR gene, specifically in exon 21 - in non-small-cell lung cancer (NSCLC) - the amino acid leucine (L) is replaced with arginine (R) at codon 858.10
Examples of insertions/deletions are as follows:
- Insertion in Exon 20 of HER2 gene in NSCLC8
- Deletion in Exon 19 of EGFR gene in NSCLC8
Examples of gene amplification/fusion are as follows:
- Amplification in HER2 gene (ERBB2) in breast cancer11
- Amplification in N-MYC in neuroblastoma12
- BCR-ABL fusion in chronic myeloid leukemia (CML)13
- ETV6-RUNX1 fusion in acute lymphocytic leukemia14
- EWSR1-FLI1 fusion in Ewing sarcoma, a bone and soft tissue cancer15
- TMPRSS2-ERG fusion in prostate cancer16
- ALK and ROS1 fusion in NSCLC17
mRNA expression analysis
This test measures the activity (expression) levels of genes in cancer cells by looking at the messenger RNA (mRNA) they produce. Some of the techniques that may be used are the following:
- Reverse transcriptase qPCR
- Digital PCR
- DNA microarrays
- RNA sequencing
Example tests are as follows:
1. Oncotype Dx measures mRNA expression of 21 genes for breast cancer18
2. MammaPrint measures mRNA expression of 70 genes for breast cancer19
3. Decipher Prostate measures mRNA expression of 22 genes for prostate cancer20
4. ColoPrint measures mRNA expression of 18 genes for colon cancer21
Protein-based analysis
Cancer cells undergo several molecular and genetic changes that result in the overproduction, alteration, or loss of normal proteins. These changes result in new proteins or modified versions of existing proteins that are released into the bloodstream or other bodily fluids — these act as diagnostic or prognostic biomarkers. Some key mechanisms include:
- Overexpression of growth factors: Cancer cells often overproduce growth factors, leading to uncontrolled cell proliferation.
- Loss of tumor suppressor proteins: Tumor suppressor proteins, such as p53, are often mutated or down regulated in cancer cells, contributing to uncontrolled cell growth.
- Altered post-translational modifications: Changes in protein modifications such as phosphorylation, glycosylation, or cleavage can produce altered protein isoforms detectable in blood samples.
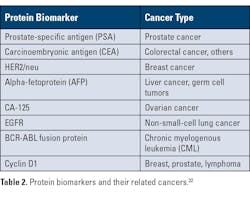
Table 2 provides examples of protein biomarkers and related cancers.
Techniques for protein-based analysis
Proteins may be detected using the following techniques:
- ELISA
- Flow cytometry
- Immunohistochemistry (IHC)
Protein expression may be detected using the following techniques:
- Mass spectrometry
- Two-dimensional gel electrophoresis
- Protein microarrays
Diagnostic imaging6
Diagnostic imaging is used to detect tumors and other abnormalities, determine the extent of disease, evaluate the effectiveness of treatment, and perform biopsies and other surgical procedures.
Imaging can be of three types:
- Transmission imaging
o X-ray
o Computed tomography scan (also called a CT scan or computed axial tomography or CAT scan)
o Bone scan
o Lymphangiogram (LAG)
o Mammogram
- Reflection imaging
o Ultrasound
- Emission imaging
o Magnetic resonance imaging (MRI)
Artificial intelligence (AI) in diagnostic imaging
Artificial Intelligence (AI) is revolutionizing diagnostic imaging.23 The benefits of AI technologies that use machine learning and deep learning are as follows:24-26
- Enhance the precision, efficiency, and personalization of diagnostic and therapeutic processes.24
- Enable image classification and analysis for superior interpretation of medical images using data-driven, automated, and assistive systems to support clinical decision-making at unprecedented levels.
- Enable automated detection algorithms that can identify patterns and abnormalities challenging for the human eye to detect.
- Use quantitative imaging biomarkers to provide deeper clinical insights to help assess disease progression, predict treatment response, and personalize patient care.
As AI technology evolves, quantitative imaging will become an integral tool for precision diagnostics and evidence-based decision-making.
Endoscopic exam6
The different types of endoscopic exams are used to examine structural abnormalities or an obstruction in different parts of the body:
- Cystoscopy to examine the bladder and urinary tract
- Colonoscopy to examine the colon and rectum
- Endoscopic retrograde cholangiopancreatography (ERCP) to examine liver, gallbladder, bile ducts, and pancreas
- Esophagogastroduodenoscopy (also called EGD or upper endoscopy) to examine esophagus, stomach, and duodenum
- Sigmoidoscopy to examine the lower one-third of the large intestine
Tumor Biopsies6
Tumor biopsies help to determine whether a tumor is malignant (cancerous) or benign. Some of the common types of biopsies are as follows:
- Endoscopic biopsy
- Bone marrow biopsy
- Excisional/wide local incision biopsy (when the entire tumor is removed) or incisional biopsy (only a portion of the tumor is removed)
- Fine needle aspiration biopsy
- Punch biopsy (involves taking a deeper sample of the skin)
- Shave biopsy (involves removing the top layers of skin by shaving it off)
- Skin biopsy
Limitations of tumor biopsies27,28
- Tissue biopsies are an invasive method and difficult to collect for some anatomical sites.
- They provide very limited information on the intratumoral and intermetastatic genetic heterogeneity and the genetic and epigenetic changes that occur with disease progression. Thus, the therapeutic decisions must be based on historical tissue biopsy results, which may be suboptimal.
- They cannot identify any lesions in different locations.
- Surgical biopsies:
o Cannot be repeated or done frequently.
o Dependent on patient’s age and health condition.
o Involve high cost.
o Can cause harmful clinical complications.28 Depending on the tissue biopsy procedure, patients may experience excessive bleeding (hemorrhage), infection, puncture damage to nearby tissue or organs, and skin numbness around the biopsy site.29
o However, tumor biopsies have limitations that can be circumvented by liquid biopsies.
o However, tumor biopsies have limitations that can be circumvented by liquid biopsies.
Tumor biopsy limitations can be circumvented by liquid biopsies. Liquid biopsies are based on the molecular profiling of biofluids and thus are able to overcome some of the above-mentioned limitations posed by tissue biopsy.27
Liquid biopsies
Advantages of liquid biopsy tests are as follows:
- Liquid biopsy is minimally invasive and hence does not pose the challenges to the patient that invasive surgical tissue biopsies may cause.
- Specimens for liquid biopsy are body fluids and can be collected frequently.
- Analysis of liquid biopsy tests can provide various information in real-time including mutations in DNA, copy number alterations (CNAs) of crucial genes, transcriptome/proteome profiling, epigenetic alterations, metabolite profiling, etc. This analysis provides crucial longitudinal information and data for more accurate diagnosis by the pathologists regarding both primary and metastasized tumors.30 Additionally, this information helps the pathologist understand the state of the tumor, see genetic and epigenetic changes occurring in the tumor with progression, and see possible metastatic alterations, allowing proper therapeutic management of the cancer patient.28
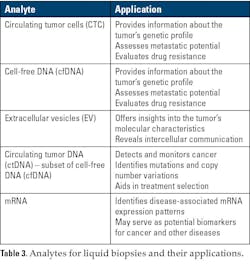
The most common methods that have been used for liquid biopsy are traditional qPCR mutational testing, droplet digital PCR (ddPCR), and next-generation sequencing (NGS).33 Newer technologies such as nanotechnology are being developed for liquid biopsy applications and have shown good promise.33,34 Table 3 provides examples of a few analytes for liquid biopsies and their applications.31
List of FDA approved tests are:32
The list of FDA approved liquid biopsy tests are as follows:
Ø Cell Search Circulating Tumor Cell (CTC) Test detects CTCs. It’s used to predict the likely outcome for people with metastatic breast, prostate, or colon cancer.
Ø Cobas EGFR Mutation Test v2 detects ctDNA. It detects a mutation on the EGFR gene in non-small cell lung cancer (NSCLC).
Ø Guardant360 CDx detects ctDNA.
Ø FoundationOne Liquid CDx detects ctDNA.
Guardant360 CDx and FoundationOne Liquid are approved both for companion diagnostics and general tumor profiling.
Treatment of cancer
Cancer is treated in several ways and is dependent on the patient’s medical condition, age, health, lifestyle, and the type and stage of cancer. Possible treatment options are chemotherapy, radiation therapy, surgery, and biological therapies.35
Owing to a better understanding of cancer as an evolving, heterogeneous disease, cancer treatment has progressed from radical surgeries and conventional chemotherapy to highly personalized approaches like precision oncology,36 targeted therapies, and immunotherapies, including CAR-T cells.37 This has led to more effective, less toxic treatments focused on the patient's specific biomarkers and the tumor's genomic characteristics.
Conclusion
There have been continuous advancements in the techniques and technologies in oncology, marked by advancements in early detection, targeted therapies, and immunotherapies. Immunotherapies that harness the body's own immune system to fight cancer (such as checkpoint inhibitors); CAR T-cell therapy;37 personalized mRNA vaccine;38 new drug delivery modalities like antibody drug conjugates (ADC);39 robotic assisted surgery;40 and combination therapies41 where different types of immunotherapies, targeted therapies, and traditional treatments may be used to enhance anti-tumor responses and overcome resistance are all transforming oncology.
This evolution is leading to highly personalized, precise therapies tailored to a patient’s tumor profile. Advancements in immunology, genomics, and technology like nanotechnology42 and artificial intelligence43 are driving this new era of precision oncology, improving both patient outcomes and quality of life. There is hope that in the near future, cancer will no longer be considered a challenging disease by healthcare professionals and patients.
References
- NCI Dictionary of Cancer Terms. Oncology. National Cancer Institute. Accessed October 3, 2025. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/Oncology.
- Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871.
- Dash BC, El-Deiry WS. Cell cycle checkpoint control mechanisms that can be disrupted in cancer. Methods Mol Biol. 2004;280:99-161. doi:10.1385/1-59259-788-2:099.
- AACR Cancer Progress Report 2024. Understanding the path to cancer development. American Association for Cancer Research. Accessed October 3, 2025. https://cancerprogressreport.aacr.org/wp-content/uploads/sites/2/2024/09/AACR_CPR_2024.pdf.
- About Cancer. Diagnosis and staging. National Cancer Institute. Accessed October 3, 2025. https://www.cancer.gov/about-cancer/diagnosis-staging.
- How is cancer diagnosed? Stanford Health Care. Accessed October 3, 2025. https://stanfordhealthcare.org/content/shc/en/medical-conditions/cancer/cancer/cancer-diagnosis.html/.
- Tumour markets. The Doctors Laboratory. Accessed October 3, 2025. https://www.tdlpathology.com/specialties/tumour-markers/.
- Pao W. Ladanyi M. Detecting gene alterations in cancers. My Cancer Genome. Updated May 26, 2019. Accessed October 3, 2025. https://www.mycancergenome.org/content/page/detecting-gene-alterations-in-cancers/.
- Tangella LP, Clark ME, Gray ES. Resistance mechanisms to targeted therapy in BRAF-mutant melanoma - A mini review. Biochim Biophys Acta Gen Subj. 2021;1865(1):129736. doi:10.1016/j.bbagen.2020.129736.
- Passaro A, Mok T, Peters S, et al. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J Thorac Oncol. 2021;16(5):764-773. doi:10.1016/j.jtho.2020.12.002.
- Ellsworth RE, Ellsworth DL, Patney HL, et al. Amplification of HER2 is a marker for global genomic instability. BMC Cancer. 2008;8:297. doi:10.1186/1471-2407-8-297.
- Kaczówka P, Wieczorek A, Czogała M, et al. The role of N-Myc gene amplification in neuroblastoma childhood tumour - single-centre experience. Contemp Oncol (Pozn). 2018;22(4):223-228. doi:10.5114/wo.2018.81402.
- Ayatollahi H, Keramati MR, Shirdel A, et al. BCR-ABL fusion genes and laboratory findings in patients with chronic myeloid leukemia in northeast Iran. Caspian J Intern Med. 2018;9(1):65-70. doi:10.22088/cjim.9.1.65.
- Li Z, Zhao H, Yang W, et al. Molecular and pharmacological heterogeneity of ETV6::RUNX1 acute lymphoblastic leukemia. Nat Commun. 2025;16(1):1153. doi:10.1038/s41467-025-56229-7.
- Jo VY. EWSR1 fusions: Ewing sarcoma and beyond. Cancer Cytopathol. 2020;128(4):229-231. doi:10.1002/cncy.22239.
- Song C, Chen H. Predictive significance of TMRPSS2-ERG fusion in prostate cancer: a meta-analysis. Cancer Cell Int. 2018;18(1):177. doi:10.1186/s12935-018-0672-2.
- Mayer C, Ofek E, Fridrich DE, et al. Direct identification of ALK and ROS1 fusions in non-small cell lung cancer from hematoxylin and eosin-stained slides using deep learning algorithms. Mod Pathol. 2022;35(12):1882-1887. doi:10.1038/s41379-022-01141-4.
- Bernhardt SM, Dasari P, Wrin J, et al. Discordance in 21-gene recurrence scores between paired breast cancer samples is inversely associated with patient age. Breast Cancer Res. 2020;22(1):90. doi:10.1186/s13058-020-01327-1.
- MammaPrint- Breast Cancer Testing. Agendia. Accessed October 3, 2025. https://agendia.com/mammaprint/.
- Decipher Prostate Genomic Classifier. Veracyte. Accessed October 3, 2025. https://www.veracyte.com/decipher-prostate/.
- Agendia announces launch of ColoPrint for colon cancer prognosis and prediction. Agendia. June 1, 2012. Accessed October 3, 2025. https://agendia.com/agendia-announces-launch-of-coloprint-for-colon-cancer-prognosis-and-prediction/.
- Damodar SV, Shukla VK, Kumar V, Deshmukh MV. The importance of protein biomarkers in cancer detection- The use of specific proteins as biomarkers for early cancer diagnosis and prognosis. Afr J Biomed. 2025;28(1s);1244-1247. https://doi.org/10.53555/AJBR.v28i1S.6406.
- Giansanti D. Revolutionizing medical imaging: The transformative role of artificial intelligence in diagnostics and treatment. Diagnostics (Basel). 2025;15(12):1557. doi:10.3390/diagnostics15121557.
- Artificial intelligence in clinical medical imaging. Diagnostics. Accessed October 3, 2025. https://mdpi-res.com/bookfiles/book/9149/Artificial_Intelligence_in_Clinical_Medical_Imaging.pdf.
- Pinto-Coelho L. How artificial intelligence is shaping medical imaging technology: A survey of innovations and applications. Bioengineering (Basel). 2023;10(12):1435. doi:10.3390/bioengineering10121435.
- Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88. doi:10.1016/j.media.2017.07.005.
- Armakolas A, Kotsari M, Koskinas J. Liquid biopsies, novel approaches and future directions. Cancers (Basel). 2023;15(5):1579. doi:10.3390/cancers15051579.
- Yu W, Hurley J, Roberts D, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466-477. doi:10.1016/j.annonc.2021.01.074.
- Ashworth, TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Australas. Med. J. 14, 146–149 (1869).
- Lone SN, Nisar S, Masoodi T, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022;21(1):79. doi:10.1186/s12943-022-01543-7.
- Noor J, Chaudhry A, Noor R, Batool S. Advancements and applications of liquid biopsies in oncology: A narrative review. Cureus. 2023;15(7):e42731. doi:10.7759/cureus.42731.
- Liquid Biopsy. Cleveland Clinic. Updated August 11, 2022. Accessed October 3, 2025. https://my.clevelandclinic.org/health/diagnostics/23992-liquid-biopsy.
- Alexandrou G, Mantikas KT, Allsopp R, et al. The evolution of affordable technologies in liquid biopsy diagnostics: The key to clinical implementation. Cancers (Basel). 2023;15(22):5434. doi:10.3390/cancers15225434.
- Goswami S, Samanta P, Adhikari MD. Nanobiotechnology: A smart platform of the future transform liquid biopsy era. J Liq Biopsy. 2024;3:100137. doi:10.1016/j.jlb.2024.100137.
- Cancer Treatment. Stanford Health Care. Accessed October 3, 2025. https://stanfordhealthcare.org/medical-conditions/cancer/cancer/cancer-treatment.html.
- Avci CB, Bagca BG, Shademan B, et al. Precision oncology: Using cancer genomics for targeted therapy advancements. Biochim Biophys Acta Rev Cancer. 2025;1880(1):189250. doi:10.1016/j.bbcan.2024.189250.
- Sun D, Shi X, Li S, et al. CAR‑T cell therapy: A breakthrough in traditional cancer treatment strategies (Review). Mol Med Rep. 2024;29(3):47. doi:10.3892/mmr.2024.13171.
- Sheridan C. Individualized mRNA cancer vaccines make strides. Nat Biotechnol. 2025;43(6):833-836. doi:10.1038/s41587-025-02708-7.
- Izzo D, Ascione L, Guidi L, et al. Innovative payloads for ADCs in cancer treatment: moving beyond the selective delivery of chemotherapy. Ther Adv Med Oncol. 2025;17:17588359241309461. doi:10.1177/17588359241309461.
- Hoeppner J. Robotic cancer surgery. Cancers (Basel). 2021;13(19):4931. doi:10.3390/cancers13194931.
- Help us unlock new combination treatments for cancer patients. The Institute of Cancer Research. Accessed October 3, 2025. https://www.icr.ac.uk/support-us/our-appeals/combination-treatments.
- Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of nanotechnology in cancer diagnosis and therapy - A mini-review. Int J Med Sci. 2020;17(18):2964-2973. doi:10.7150/ijms.49801.
- Artificial Intelligence (AI) and Cancer. National Cancer Institute. May 30, 2024. Accessed October 3, 2025. https://www.cancer.gov/research/infrastructure/artificial-intelligence.
To take the test online go HERE. For more information, visit the Continuing Education tab.
About the Author

Rajasri Chandra, MS, MBA
is a global marketing leader with expertise in managing upstream, downstream, strategic, tactical, traditional, and digital marketing in biotech, in vitro diagnostics, life sciences, and pharmaceutical industries. Raj is an orchestrator of go-to-market strategies driving complete product life cycle from ideation to commercialization.