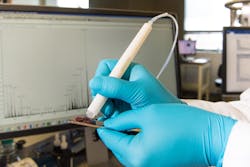
A diagnostic tool called the MasSpec Pen has been tested for the first time in pancreatic cancer patients during surgery, according to a news release from the University of Texas at Austin.
The device is shown to accurately identify tissues and surgical margins directly in patients and differentiate healthy and cancerous tissue from banked pancreas samples. At about 15 seconds per analysis, the method is more than 100 times as fast as the current gold standard diagnostic, frozen section analysis, according to the university. The ability to accurately identify margins between healthy and cancerous tissue in pancreatic cancer surgeries can give patients the greatest chance of survival.
The results, by a team from The University of Texas at Austin and Baylor College of Medicine, are published in the Proceedings of the National Academy of Sciences.
The most common type of pancreatic cancer, pancreatic ductal adenocarcinoma, spreads rapidly and is highly lethal, with a five-year survival rate of 9% for all stages. The most effective treatment option is surgical removal of the tumor.
For this study, the investigators first used the MasSpec Pen to analyze 157 banked human pancreatic tissues to develop and evaluate the technology in the laboratory for pancreatic cancer. Then, the investigators moved the system to the operating room at Baylor St. Luke’s Medical Center in Houston, which is affiliated with Baylor College of Medicine, where the surgeons tested the technology in 18 pancreatic surgeries.
The pen has been tested in more than 150 human surgeries to date, including for breast and thyroid, and results of those additional tests will be submitted for publication soon.