Proper patient preparation, specimen collection, and sample handling are critical to quality care
Garbage In, Garbage Out (GIGO), as the saying goes. This adage has been applied in a universal manner in addressing human errors. It certainly applies to establishing laboratory procedures that ensure care in managing the pre-analytical phase of laboratory testing. Sixty years ago, many common laboratory tests were performed manually, and thus were prone to inaccuracy and analytical mistakes. Today’s advanced technology places laboratory science in a highly automated and quality-focused environment that ensures accurate testing processes.
Total Testing Process (TTP)
Medical errors are the third leading cause of death in the U.S. The laboratory’s contribution to this major healthcare concern is only 0.33 percent.1 While this number appears small, laboratory errors do occur, not always resulting is death, but nevertheless having an important impact on patient care. As clinical laboratory scientists, we must make every effort to produce accurate test results.
One of the first efforts (1947) in laboratory quality management involved the collection of survey results where six common analyte samples were shared with a number of laboratories. The results pointed to wide and significant variation.2 In later years (1997) the concept of "brain-to-brain turnaround time" was introduced by George Lundberg and later modified to "brain-to-brain loop."3 This process included nine steps involving laboratory testing: 1) ordering, 2) collection, 3) identification, 4) transportation, 5) separation, 6) analysis, 7) reporting, 8) interpretation, and 9) action.1 This became the basis for the concept of the Total Testing Process (TTP), to include pre-analytic, analytic, and post-analytic phases of testing. Over the past decades, as a result of intense quality management programs, laboratory errors have been reduced by as much as 75 percent.1
While efforts were initially focused on the analytical part of laboratory testing, it became clear that the pre-analytical and post-analytical phases of testing were just as, if not more, important in providing quality services. A number of studies have been done that have looked at the accuracy of laboratory testing. In general, error rates for each phase range from 46 percent to 68 percent for pre-analytical, 7 percent to 13 percent for analytical, and 18 percent to 47 percent for post-analytical.4 Since almost 70 percent of all laboratory errors reside within the pre-analytical phase, laboratories began to focus on preemptive practices that would minimize, and hopefully avoid, common errors.
Pre-pre-analytic and pre-analytic phases
The pre-pre-analytic phase has been has been added as part of the initial patient encounter process.5,6 As noted, this is where most laboratory errors occur. Table 1 identifies some areas of common errors. These errors may cost a moderate-sized hospital just over $1 million a year in quality assurance investigations, blood redraws, repeat testing, and management oversight.7
One of the most common errors is ordering the wrong test.5,8 With the ever-expanding clinical laboratory test menu, ensuring that the right test is ordered can be somewhat daunting. Some examples: factor V and factor V Leiden; 25-hydroxyvitamin and 1,25-dihydroxyvitimin D; and thyroid tests. With a huge influx of information overload, the addition of new tests, the pressure to see more patients, and the expanding use of cutting-edge molecular diagnostics create numerous opportunities to make mistakes in laboratory test orders.
Other pre-analytic variables
One of the least invasive procedures a patient may endure is the phlebotomy, yet it may be a significant source of poor patient care:8-13
- Proper patient identification is mandatory. It is also useful to understand the patient’s health status, which may have an influence on test results.
- Regulations require that at least two patient identifiers be obtained, usually spelling of their name and their date of birth.
- In addition, proper labeling of the specimen is also critical (patient’s name, hospital number or date of birth, time and date of collection, phlebotomist’s name).
- Fasting status.
- Patient diagnosis.
- Correct test ordered.
- Physiologic factors
- Fasting, especially prolonged fasting, may cause increases in amino acids, bilirubin, ketones, growth hormones, fatty acids, and triglycerides. Decreases may be observed in assays for glucose, high density lipoprotein (HDL), insulin, T3, and lactate dehydrogenase (LD). Fatty meals may increase potassium, triglycerides, alkaline phosphatase, and 5-hydroxyindoleacetic acid (5-HIAA). Meat, fish, iron, horseradish, and some bismuth-based antacids may render a false positive in stool occult blood tests.
- Diets
- Vegetarians, especially long-term vegetarians, may show decreased levels of low density lipoprotein (LDL), very low density lipoprotein (VLDL), phospholipids, cholesterol, triglycerides, and vitamin B12.
- High protein/meat diets show increased serum urea, ammonia, and urate levels.
- High protein/low carbohydrate diets (Atkins Diet) can result in increased urine ketones and serum blood urea nitrogen (BUN).
- Alcohol intake can increase triglycerides, lipoprotein, B12, lactate, and liver enzymes. Decreased levels of glucose, prolactin, and cortisol may also occur. Alcohol abuse can reflect elevation of HDL, ϒ-glutamyltransferase (GGT), urate, and mean corpuscular volume (MCV).
- Coffee/caffeine may increase glucose, plasma rennin, free fatty acids, and catecholamine concentrations. Increased levels of non-esterified fatty acids may interfere with albumin-bound drug and hormone measurements. Studies suggest that caffeine may decrease platelet aggregation and increase vitamin D levels.14,15
- Other factors: Bananas, pineapples, tomatoes, and avocadoes may elevate urine 5-HIAA. Cholesterol, triglycerides, and apoB lipoproteins may be altered in obese patients.
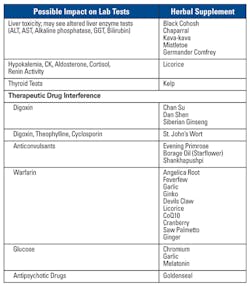
- Herbal supplements: Herbal supplements are used globally for a variety of ailments. Numerous studies have shown how some supplements can interfere with therapeutic drugs as well as laboratory testing. Most, if not all herbal supplements are not regulated by the FDA; thus their co-activity with other agents may not be fully appreciated. Various Chinese herbs have been shown to affect some laboratory test values. In addition, heavy metal poisoning has been reported based on elevated lead and zinc protoporphyrin levels. Table 2 lists some common supplements.11,12
- Exercise: Exercise may increase free fatty acids, lactate, creatine phosphokinase (CK), creatinine, pyruvate kinase, aspartate aminotransfrerase (AST), lactate
dehydrogenase (LD), aldostrone, cortisol, HDL, prolactin, uric acid, bilirubin, platelets, WBC, and ammonia. Cholesterol and triglycerides may decrease. - Posture: Plasma rennin activity and calcium may be lower when supine. Changing from supine to upright can alter hemoglobin, hematocrit, RBC, WBC, calcium, protein, and lipid levels.
- Stress: Hormones tend to increase with stress. Total cholesterol may increase, while HDL may decrease.
- Exercise: Exercise may increase free fatty acids, lactate, creatine phosphokinase (CK), creatinine, pyruvate kinase, aspartate aminotransfrerase (AST), lactate
- Blood collection
- Tourniquet: Prolonged tourniquet application (hemoconcentration > one minute) may affect potassium, magnesium, albumin, protein, serum enzymes, coagulation factors, iron, ammonia, calcium, cholesterol, and
triglyceride levels. - Decontamination: Alcohol that has not dried ( it needs 30 to 60 seconds) can destroy red cells. Iodine may elevate phosphate, uric acid, and potassium.
- Order of draw: First to draw are blood cultures followed by light blue tubes (Na citrate), red (SST), green (heparin), lavender (versene), yellow (ACD), and finally gray (K oxalate/Na fluoride) tubes.
- Wrong tubes: Using the wrong anticoagulant in collection can alter test results.
- Improper mixing: Gentle mixing, using complete 180o inversions, is critical. For serum separation tubes (SST), inverting five times, allowing to sit 30 minutes, and then centrifuging (1,000-1,300 RCF) in a swing bucket for 10 minutes provides a proper specimen for analysis.16
- Tourniquet: Prolonged tourniquet application (hemoconcentration > one minute) may affect potassium, magnesium, albumin, protein, serum enzymes, coagulation factors, iron, ammonia, calcium, cholesterol, and
- Interfering substances
- Tobacco: Hemoglobin, carboxyhemoglobin, catecholamines, glucose, lactate, growth hormone, cholesterol, triglycerides, LDL, cortisol, WBC, RBC, and MCV may be elevated. Decreased levels of vitamin B12, HDL, IgA, IgG, IgM, and sperm counts/motility are also seen.
- Hemolysis: As red cells break apart, potassium, phosphorus, ALT, LD, CK, coagulation factors, magnesium, iron, sodium, HDL, triglycerides, and cholesterol levels may be altered.
- Lipemia: When triglycerides exceed 300 mg/dL, lipemia occurs, causing difficulties in measuring hemoglobin, white blood cells, and platelets. Any assay using spectral analysis may be affected by the abnormal light scatter caused by lipemic particles.16
Knowing the variables
Proper preparation of the patient regarding fasting needs as well as careful phlebotomy procedures are essential to ensuring accurate laboratory testing results. General practice is to have the patient fast twelve hours prior to blood draw, especially when testing for a lipid profile and glucose tests. There is also evidence that fasting for creatinine levels should be considered, especially when high protein/meat diets are consumed.17 In addition, patients should avoid alcohol, tobacco, physical stress, and caffeine. Having the patient sit for fifteen minutes prior to the blood draw and performing it in the morning (7-11 AM) is ideal.13
While all pre-analytic variables cannot be eliminated, phlebotomists and technical staff need to be made aware of the many variables that can impact laboratory testing accuracy. Up-to-date blood collection procedures should provide guidance in addressing potential critical outcomes in the pre-analytical phase of testing. Establishing quality assurance monitors for selected possible pre-analytic errors can help identify opportunities that can be addressed, thus minimizing the risk of adverse outcomes.
REFERENCES
- Ghaedi M, El-Khoury J. The leading cause of error in laboratory medicine. Clin Lab News.2016. https://www.aacc.org/publications/cln/articles/2016/july/preanalytical-variation-the-leading-cause-of-error-in-laboratory-medicine.
- Belk WP, Sunderman FW. A survey of the accuracy of chemical analysis in clinical laboratories. Am J Clin Pathol. 1947;17(11):853-861.
- Lundberg GD. Acting on significant laboratory results. JAMA. 1981;245:1762-1763.
- Hammerling JA. A review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44.
- Laposata M, Dighe A. "Pre-pre’’ and ‘‘post-post’’ analytical error: high-incidence patient safety hazards involving the clinical laboratory. Clin Chem Lab Med. 2007;45(6):712–719.
- Hawkins R. Managing the pre- and post-analytical phases of the total testing process. Ann Lab Med. 2012;32(1):5-16.
- Kaushik N, Green S. Pre-analytical errors: their impact and how to minimize them. MLO. 2014;46(5):22-26.
- Lifshitz MS. Preanalysis. Henry’s Clinical Diagnosis and Management by Laboratory Methods, 23rd ed. McPherson & Pincus, eds. 2016; pp. 20-32.
- Finnagan K. Pre-analytical variables and patient safety. ASCP presentation, San Francisco. April 28, 2015.
- Young DS, Bermes EW, Haverstick DM. Specimen collection and other preanalytic variables. Tietz: Fundamentals of Clinical Chemistry, 6th ed. Burtis, Ashwood, Bruns, eds. 2008; pp 42-62.
- Dasgupta A, Bernard DW. Herbal remedies: effects on clinical laboratory tests. Arch Pathol Lab Med. 2006;130(4):521-528.
- Dasgupta A. Review of abnormal laboratoru test results and toxic effects due to use of herbal medicines. Am J Clin Pathol. 2003;120(1):127-137.
- Irjala KM, Gronroos PE. Preanalytical and analytical factors affecting laboratory results. Ann Med. 1998;30(3):267-272.
- Natella F, Nardini M, Belelli F, et al. Effect of coffee drinking on platelets: inhibition of aggregation and phenols incorporation. Br J Nutri. 2008;100:1276-1282.
- Al-Othman A, Al-Musharaf S, Al-Daghri NM, et al. Tea and coffee consumption in relation to vitamin D and calcium levels in Saudi adolescents. Nutre J. 2012;11:56
- Kurec AS. Can the type of blood collection tubes used be a source of lab error? MLO. 2016;48(10):18.
- Kurec AS. Answering your questions. Lipemia and hyperleukocytosis can lead to CBC errors. MLO. 2016;48(7):64.
- Lab Tests Online. www.labtestsonline.org.
- Anderson L. 18 Herbal supplements with risky drug interactions. Drugs.com. 2014; https://www.drugs.com/slideshow/herb-drug-interactions-1069.
Anthony Kurec, MS, H(ASCP)DLM, is Clinical Associate Professor, Emeritus, at SUNY Upstate Medical University in Syracuse, New York. He is also a member of the MLO Editorial Advisory Board.
About the Author

Anthony Kurec, MS, MASCP, MLT, H(ASCP)DLM
is Clinical Associate Professor, Emeritus, at SUNY Upstate Medical University in Syracuse, NY. He is also a member of the MLO Editorial Advisory Board.