The College of American Pathologists has published new evidence-based guidelines to enhance the diagnosis and subtyping of amyloidosis, aiming for earlier and more accurate detection to improve patient care, according to an announcement.
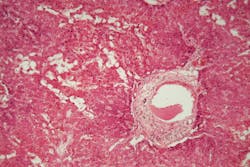
Amyloidosis is a rare but serious disease caused by the buildup of abnormal amyloid deposits in the body. The new guideline aims to support earlier and more accurate diagnosis, which is critical for effective treatment but has often been challenging for laboratories and pathologists.
A multi-disciplinary panel of experts reviewed more than 4,000 studies to develop recommendations on the most accurate methods for detecting amyloid, identifying fibril protein type, and evaluating sample types. The guideline provides pathologists with clear direction on appropriate testing and subtyping of amyloid-positive specimens.
Key recommendations:
- Cytology: May be used for fat pad screening; less invasive but limited for subtyping.
- Congo Red Stain: Remains the standard for amyloid detection; other stains should be validated.
- Fluorescence Microscopy: May improve Congo red sensitivity where available.
- Protein Typing: Mass spectrometry recommended for greatest accuracy and sensitivity.
Good practice recommendations:
- When specimens are received for detection of systemic amyloidosis, pathologists should evaluate for the presence of amyloid using validated method(s), and the validated method(s) used should be identified in the pathology report.
- If a clinical concern for amyloidosis persists after a negative biopsy from a surrogate site but the potentially affected organ(s) was not sampled, then suggesting biopsy of the potentially affected organ(s) and/or recommending suitable archived specimen(s) to evaluate is appropriate.
- In patients with amyloidosis being considered for therapy, pathologists should determine the fibril protein type.
The CAP said they will reassess the guideline every five years or sooner, as warranted by advancements in research or clinical practice.