Isolated anti-DFS70 autoantibodies: Negative disease correlation with a positive impact
Earning CEUs:
For a printable version of the July CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
JULY LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss the utility of ANA autoantibody testing in relation to SARD diseases.
2. Recall the pattern and antibody specificity for confirmatory testing and describe its utility.
3. Recall the committee for the standardization and diagnostic use of the DFS ANA pattern.
4. Discuss the findings that an outside laboratory found when it adopted the use of confirmatory testing when finding DFS patterns on ANA tests.
The presence of antinuclear autoantibodies (ANA) is one of the key diagnostic criteria of systemic autoimmune rheumatic diseases (SARD), such as systemic lupus erythematosus (SLE), Sjogren’s syndrome, systemic sclerosis, dermatomyositis/polymyositis (DM/PM), and mixed connective tissue diseases (MCTD). Indirect immunofluorescence assays (IFA) using human epithelial (HEp-2) cells are the American College of Rheumatologists recommended “gold standard” for ANA screening as this substrate provides a variety of more than 100 native autoantigens including proteins, DNA, and ribonucleoproteins.1
Relevance of anti-DFS70 testing
The dense fine speckled (DFS) nuclear pattern is one of the most common IFA patterns encountered in the ANA screening routine of clinical diagnostic laboratories, often occurring in very high titers. The autoantibodies producing this pattern target the DFS protein of 70 kDa (DFS70), which is identical to the Lens Epithelium-Derived Growth Factor or transcription co-activator p75 (LEDGFp75). DFS70/LEDGFp75 confers cell protection by regulating transcription of stress-related genes and is relevant to the pathophysiology of AIDS, cancer, autoimmunity, and inflammatory conditions. Anti-DFS70 autoantibodies might play protective, pathogenic, or sensor roles.2
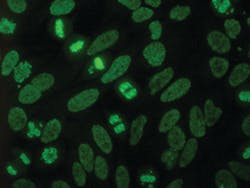
The International Consensus on ANA Patterns (ICAP) committee has classified the DFS pattern as “AC-2,” a competency level recognition pattern, defined by a dense and heterogeneous speckled staining in the nucleoplasm of interphase cells (sparing the nucleoli) and the metaphase chromosomal plate.3 (Figure 1)
Recognition of this pattern on HEp-2 substrates is challenging as it can be confused with other nuclear patterns or may occur in the context of another clinically relevant ANA, and because IFA interpretation is dependent on technician expertise.
Thus, a positive DFS IFA result has to be followed by a monospecific immunoassay (e.g., ELISA, ChLIA, immunoblot) to accurately confirm the presence of anti-DFS70 autoantibodies, as recommended in diagnostic algorithms.4 The clinical significance of anti-DFS70 autoantibodies is not clear as there is no known disease specificity for this autoantibody.2
Regardless of the detection method, DFS ANA and/or anti-DFS70 antibodies have been detected at elevated frequency in apparently healthy individuals (0–21.6 percent), but also in routine ANA screening cohorts (0.3–16.6 percent), and various non-SARD inflammatory and neoplastic conditions (3.3–71.4 percent; for example, Vogt-Harada syndrome, atopic dermatitis, psoriasis, interstitial cystitis, Hashimoto’s thyroiditis, ocular diseases, chronic fatigue syndrome, asthma, or prostate cancer). In contrast, they are rare in patients with SARD (0–28.6 percent), showing an overall frequency of only 2.8–4.5 percent, which is remarkably lower than in healthy individuals and control cohorts. Anti-DFS70 reactivity in SARD is usually accompanied by additional SARD-related antibodies, while isolated anti-DFS70 reactivity in SARD reportedly amounts to only 0.5–0.7 percent.5,6
Thus, antibodies to DFS70 are increasingly regarded as a potential negative predictive biomarker for excluding the diagnosis of SARD, particularly in the absence of clinically relevant ANA. This is supported by studies reporting on healthy individuals with isolated anti-DFS70 reactivity who did not develop SARD within a follow-up of three to four years (“benign autoimmunity”), and by a likelihood ratio (LR+) for the absence of SARD of 10.9 ascribed to isolated reactivity to DFS70.7
Overall, a few recent surveys have examined the prevalence of DFS ANA in sera submitted for routine ANA screening, but only some of these used anti-DFS70 assays to confirm the antibodies’ specificity in all or in just a subset of tested samples. Considering also the diverse composition of the screened cohorts as well as the differences in assays and IFA interpretation, the currently available data still requires more research. But there is strong evidence that isolated anti-DFS70 positivity—in the absence of disease-associated ANA—indeed correlates negatively with a SARD diagnosis. Anti-DFS70 testing can already help prevent unnecessary referrals to tertiary care specialists and potentially unnecessary (toxic) treatment. Also, there are economic implications for the healthcare system.
In a Spanish cohort of 181 patients a proposed algorithm that included anti-DFS70 testing saved approximately $70k of combined lab costs and outpatient clinic visits across all patients.8 From a U.S. perspective, this might be an underestimation as the healthcare expenditures are significantly greater in the U.S. as compared to Spain or Europe, in general.
A practical community hospital experience report
In order to illustrate the practical impact of anti-DFS70 testing, John B. Carter, MD and Sara Carter, MT(ASCP)SM,SI from Lexington Medical Center Laboratory (LMC) in West Columbia, SC discuss below their experience in a community hospital setting. LMC is one of the first testing facilities in the U.S. to adopt use of the ICAP system.
Developing an understanding for anti-DFS70
For many years the laboratory noted frequent, often high-titer (>10,240) speckled ANAs having a negative 6-test anti-Extractable Nuclear Antigens (anti-ENA)/anti-DNA profile and no clinical features of autoimmune rheumatic disease. When a mitotic-rich HEp-2 cell substrate became available, the laboratory noted many of these speckled ANAs to have strong fluorescence of mitotic figures (metaphase plates).
Recent technical advances enabled the community hospital immunology lab to significantly upgrade testing services. In an ongoing study of over 13,000 cases of routine DFS70 ANA testing it was noted that DFS, AC-2 was a common ANA pattern (21 percent of positive ANAs) that was readily apparent by IFA on a high-mitotic substrate, with a high percentage (47 percent) of these DFS patterns showing anti-DFS70 specificity by a line immunoblot assay. The majority of these DFS70-positive ANAs (76 percent) were not associated with other antigen specificities on a 16-test anti-ENA profile that includes anti-DFS70 specificity, or with significant clinical findings on chart review, and were termed an “isolated DFS70” ANA.
To date, with up to a 42-month follow-up, no patient with an isolated DFS70 ANA has developed findings of autoimmune rheumatic disease. However, several patients had sero-positive rheumatoid arthritis.9
A case report
A sentinel isolated DFS70 ANA case is illustrated by a 42-year-old woman who had a “homogeneous” ANA at 1:640 titer on a 2005 physical exam, prompting extensive testing and long-term follow-up to rule out development of systemic lupus. Crithidia anti-DNA and a 6-test anti-ENA profile were negative. While further studies were negative, there were ongoing concerns of a “pre-SLE” syndrome, and she continued to be monitored for evidence of developing rheumatic disease.
Repeat testing in 2017 showed a DFS, AC-2 ANA at 1:1280 titer. Anti-DFS70 was strongly positive. The 16-test anti-ENA profile was negative for other antigen specificities and she had no significant clinical findings. She had been monitored for evidence of SARD because of concerns due to the previously positive ANA. The significance that the high-titer isolated anti-DFS70 ANA did not reflect autoimmune rheumatic disease was explained to the patient and her physician.
Patients with a “non-isolated DFS70” ANA—where one or more additional antigen specificities were positive on an expanded anti-ENA profile—had clinical findings appropriate to the other anti-ENA markers such as a variety of rheumatic disorders, including systemic lupus, mixed connective tissue disease, Sjogren’s syndrome, autoimmune myositis, and systemic sclerosis. Thus, it was apparent that other SARD-related antibodies in the anti-ENA profile, not anti-DFS70, reflected the clinical course.
Assay considerations at LMC
While use of mitotic-rich ANA substrate slides in the identification of DFS ANAs is readily adaptable to conventional or manual ANA testing methods, the laboratory found that semi-automated slide preparation and computer-assisted microscopy to be a significant aid in managing a moderate to high volume ANA workload (averaging 600 ANA tests/month) and to enhance slide review by several viewers.
Computerized storage of ANA patterns has significant advantage, permitting long-term storage of ANA results and easy transmission of ANA patterns to interested physicians and consulting laboratories.10
Only 40 percent of ANAs with a DFS, ICAP AC-2 pattern showed DFS70 ANA specificity—evidence that the fluorescent microscopic DFS IFA pattern alone is not sufficient to “call” a DFS70 ANA, as 60 percent of DFS ANA patterns did not show anti-DFS70 specificity, but reflect antibody to a variety of known and unknown antigens, mixed ANA patterns, or a misreading of a homogeneous ANA pattern.11 A mono-specific anti-DFS70 test is necessary to confirm that anti-DFS70 is a component of a positive ANA. However, a single stand-alone DFS70-specific test is not a sufficient confirmation of an isolated DFS70 ANA, as 27 percent (1/4) of DFS70 ANAs in this study contained antibodies to one or more other anti-ENAs, which often reflected active rheumatic disease.9
A 6-test anti-ENA profile is not a sufficient overview of possible ANA-related autoantibody specificities, and while several expanded anti-ENA profiles and methods are available, the laboratory found that an expanded profile containing antigens specific for anti-Sm, U1RNP, SSA, SSB, Ro-52, PM-Scl100, PCNA, Chromatin, Histones, and Ribosomal P-Protein covers the majority of systemic lupus and connective tissue disease related antibodies, as well as the most common autoimmune myositis (Jo-1, PM-Scl) and systemic sclerosis (Scl-70, CENP-B) related antibodies.12 Clinical findings suggestive of active myositis, including interstitial lung disease or limited and diffuse forms of systemic sclerosis warrant further specific testing.
Computer-formatted templates
As immunology-related laboratory tests are ordered, and results are interpreted by a variety of healthcare providers and physician specialists, interpretive comments are often helpful in supporting optimal use of these specialty tests.
The following computer-formatted templates have proven useful, and are easily tailored to fit individual case results:
- In the case of a positive (titer >40) ANA: “Suggest anti-DNA and expanded anti-ENA profile (that includes anti-DFS70) if this is a new finding. Active rheumatic disease is rare in untreated patients with an ANA titer <160.”
- In the case of an isolated DFS70 ANA: “An isolated DFS70 ANA result rarely reflects systemic autoimmune rheumatic disease in the absence of other positive ENA’s or significant clinical features.”
- In the case of a DFS70 ANA with other anti-ENA specificities: “Positive anti-ENA-specificities other than DFS70, as noted by elevated levels of anti-____ antibody(ies) in this profile, may have clinical relevance related to these antibodies.”
The take-home messages
The takeaway after 3.5 years of anti-DFS70 testing at LMC is that the detection and identification of “isolated” DFS70 ANAs should be part of ANA testing labs as these test results directly affect a significant number of patients. Approximately five percent of positive, even high-titer ANAs are due to an isolated anti-DFS70 that is rarely associated with active rheumatic disease, and these patients can be reassured that they do not have serological evidence of active rheumatic disease, and that further investigations are unnecessary.
The following points will be important in achieving this goal and in an overall strengthening of the ANA testing service:
- Increased familiarity and use of the ICAP system will be very useful and relevant to classification of all ANA patterns and to communication with other medical specialists.
- A mitotic-rich HEp-2 ANA substrate is essential for reliable recognition of the DFS, ICAP AC-2 pattern as well as other mitotic ANA patterns.
- Anti-DFS ANA patterns warrant follow-up testing for anti-DFS70 specificity as the DFS ANA pattern alone is not DFS70 specific. Many speckled ANAs are associated with other ENA specificities which often reflect autoimmune disease.
- ANA follow-up testing should use an expanded anti-ENA panel that includes a specific anti-DFS70 test. Other anti-ENAs in addition to an anti-DFS70 are common and are often clinically significant.
- An isolated DFS70-ANA rarely reflects SARD, in the absence of significant clinical findings, if the expanded anti-ENA profile is otherwise negative. This offers explanation of the positive ANA and reassurance that an autoimmune process is unlikely and further work-up unnecessary.
- An isolated DFS70 ANA does not “exclude” the diagnosis of autoimmune rheumatic disease. It is merely an ANA result that does not support the diagnosis and should not be considered a “point” in favor of a SARD diagnosis in clinical diagnostic algorithms.
- No single ANA or anti-ENA test result is independently diagnostic or exclusionary of autoimmune rheumatic disease. These test results are “another piece of evidence” in an overall evaluation of a possible autoimmune diagnosis, and correlation with clinical features and other test results is essential.
For a printable version of the July CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
REFERENCES
- Position Statement: Methodology of Testing for Antinuclear Antibodies. Atlanta, GA: American College of Rheumatology (2015). https://www.rheumatology.org/Portals/0/Files/Methodology%20of%20Testing%20Antinuclear%20Antibodies%20Position%20Statement.pdf
- Ochs RL, Mahler M, Basu A, et al. The significance of autoantibodies to DFS70/LEDGFp75 in health and disease: integrating basic science with clinical understanding. Clin Exp Med (2016) 16:273–93. doi:10.1007/s10238-015-0367-0.
- Chan EK, Damoiseaux J, Carballo OG, et al. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014-2015. Front Immunol (2015) 6:412. doi:10.3389/fimmu.2015.00412.
- Bizzaro N, Pesente F, Cucchiaro F, et al. Anti-DFS70 antibodies detected by immunoblot methods: a reliable tool to confirm the dense fine speckles ANA pattern. J Immunol Methods (2016) 436:50–3. doi:10.1016/j.jim.2016.06.008.
- Shovman O, Gilburd B, Chayat C, et al. Prevalence of anti-DFS70 antibodies in patients with and without systemic autoimmune rheumatic diseases. Clin Exp Rheumatol (2018) 36:121–6.
- Seelig CA, Bauer O, Seelig HP. Autoantibodies against DFS70/LEDGF exclusion markers for systemic autoimmune rheumatic diseases (SARD). Clin Lab (2016) 62:499–517. doi:10.7754/Clin.Lab.2015.150905.
- Fitch-Rogalsky C, Steber W, Mahler M, et al. Clinical and serological features of patients referred through a rheumatology triage system because of positive antinuclear antibodies. PLoS One (2014) 9:e93812. doi:10.1371/journal.pone.0093812.
- Gundín S, Irure-Ventura J, Asensio E, et al. Measurement of anti-DFS70 antibodies in patients with ANA-associated autoimmune rheumatic diseases suspicion is cost-effective. Auto Immun Highlights. 2016 Dec;7(1):10. doi: 10.1007/s13317-016-0082-1.
- Carter J, Carter S, Saschenbrecker S, et al. Recognition and Relevance of Anti-DFS70 Autoantibodies in Routine Antinuclear Autoantibodies Testing at a Community Hospital. Front. Med. (2018) https://doi.org/10.3389/fmed.2018.00088.
- Ricchiuti V, Adams J, Hardy D, Et al. Automated processing and evaluation of anti-nuclear antibody indirect immunofluorescence testing. Frontiers in Immunology. 2018; 9#927, 1-11.
- Carbone T, Pafundi V, Tramontano G, et al. Prevalence and serological profile of anti-DFS70 positive subjects from a routine ANA cohort. Scientific Reports 9, Article number: 2177 (2019).
- Carter S, Carter J, Richardson D, et al. The Euroimmun ANA Profile 1 as an expanded ENA profile. Am J Clin Pathol. 2014; 142:A192.
About the Author

John B. Carter, MD
served as Clinical Laboratory Director of Lexington Medical Center (LMC) for 30 years and currently serves as Clinical Lab consultant at LMC.

Sara Carter, MT (ASCP)SM,SI
was co-founder and Technical Director of Lexington Medical Laboratories for 35 years, an LMC affiliate.

Oliver Sendscheid, PhD
serves as Scientific Affairs Director for the U.S. subsidiary of EUROIMMUN.