The (r)evolution of ESR: Photometric rheology
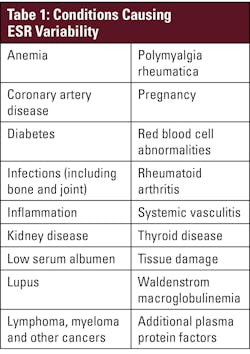
Patients suspected of having an acute or chronic inflammatory condition are often tested for inflammatory markers.1 Erythrocyte sedimentation rate (ESR), frequently referred to as a “sickness indicator,” is an established, widely used test and can be used as a general marker of inflammation2 both in acute diagnosis and monitoring of treatment and severity of chronic conditions.3 Measurement of erythrocyte sedimentation provides clinically valuable information about a patient’s condition not provided by C-reactive protein (CRP) alone. CRP is typically associated more with acute inflammation and less with cancer and chronic or diffused inflammatory conditions. While ESR should not be used alone to diagnose a medical condition, it can help determine severity of an established condition or as a measure to lead to a diagnosis in combination with other tests or procedures. ESR is commonly performed when there is a suspicion of any number of inflammatory conditions — including subclinical, acute, and chronic conditions — and can be elevated in systemic infections, orthopedic infections, bronchiolitis, giant cell arteritis, kidney, coronary, and autoimmune diseases, vasculitis, and certain cancers, among other conditions (see Table 1).2-4
Traditional ESR methods and limitations
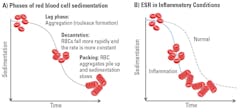
Erythrocyte sedimentation was originally observed by Polish physician Dr. Edmund Faustyn Biernacki in 1897 and further studied by Swedish hematologists and internists Dr. Robert Fahraeus and Dr. Alf Vilhelm Albertsson Westergren in the early 1900s.2,5 The presence of acute-phase plasma proteins causes an increase in RBC aggregation, and increased RBC aggregation is proportional to increased RBC sedimentation (see Figure 1). Historically, ESR has been measured with the standard Westergren test developed by Dr. Westergren in 1921. This technique relies on placing diluted blood in a narrow, vertical tube and measuring the gravitational sedimentation of RBCs over 60 minutes. Although the term sedimentation rate is used, RBCs do not fall at a constant rate; sedimentation occurs in three phases (Figure 1). The Westergren test has a 60-minute incubation to allow for full sample sedimentation and thus is subject to many environmental and operational variables, including ambient temperature fluctuations, tube angle (± 3°), operator technique, and interference from bench vibration, among others. The sample age and transport conditions prior to testing are important; CLSI and ICSH guidelines specify that Westergren ESR samples should be tested within 4 hours if stored at room temperature or 24 hours if refrigerated.6,7 A relatively short four-hour window of sample stability can be challenging for a routine lab test, especially when collection sites are widely dispersed. The Westergren method also requires significant hands-on time, exposes laboratory personnel to biohazards, and generates considerable disposable waste. Even modified Westergren methods that utilize automation to shorten testing time often retain these limitations.
Newer techniques and advantages
As clinical laboratory staffing challenges persist, it can be difficult to devote hands-on time to manual Westergren tests. In response, photometric rheology has emerged as an alternative method of ESR to improve efficiency by minimizing operator intervention, reducing turnaround time, extending sample stability, and removing subjectivity associated with Westergren. It is faster because it does not measure the final amount of sedimented RBCs but measures rouleaux formation — which represents the preliminary aggregation of RBCs in the earliest stage of sedimentation. Photometric rheology offers a direct measurement of initial RBC aggregation in the lag phase of sedimentation thus is less affected by variables such as sample age, hematocrit, vibration, or room temperature. By utilizing micro-flow cell technology, modern photometric rheology analyzers maintain a highly controlled testing environment while maintaining temperature at 37°C, reducing variability from outside sources. The extended sample stability that is afforded by photometric rheology has a multitude of practical benefits — including greater flexibility in sample transport logistics and reduced redraws — and ultimately helps ensure result reliability.
The science behind photometric rheology
Photometric rheology utilizes a measure of light transmission to directly detect rouleaux formation, which occurs during the earliest stage of the sedimentation process and ultimately determines the amount of RBC sedimentation. Within photometric rheology–based ESR analyzers, a whole blood sample is mixed, then 100 μl of sample is aspirated and pumped into a flow cell that disaggregates the RBCs. When the pump stops, light transmission through the sample is monitored as the RBCs begin to re-aggregate (see Figure 2). Initially, light transmission through the disaggregated sample is low due to impact of the surface area of many individual RBCs blocking light. As RBCs begin to re-aggregate in the rouleaux formation (akin to a “stack of coins”), the overall surface area of the RBCs is reduced, and therefore more light is transmitted through the sample. Rouleaux formation begins within seconds of the pump stopping, and photometric rheology ESR analyzers can provide results in 15–20 seconds after sample mixing. In the context of inflammation, increased rouleaux formation due to plasma fibrinogen and globulins will cause increases in light transmission that reflect changes in these acute-phase proteins associated with many inflammatory conditions. Photometric rheology ESR analyzers generate results in units of mm/hr and are correlated to the Westergren method.
Real-world stability: Evolving technology for the changing lab
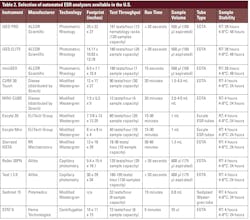
Most clinical laboratories in the United States face critical staffing and funding shortages, particularly in the hematology department. Approximately 12,000 new graduates are needed yearly to meet demand, but only about 5,000 individuals complete their training programs.8 On top of this, only 12% of medical technologists plan on staying in laboratory medicine long term.9 There is 15% turnover of medical technologists, one of the highest turnover rates of all hospital employees.10 Within this group, there is already a vacancy rate for hematology laboratory staff of 16.6% with another 16.7% planning to retire in the next 5 years.11 Many labs are turning to relatively untrained new hires to fill staffing gaps. With staffing shortages at an all-time high, reduced hands-on times, quicker assay turnaround times, and shorter training requirements are paramount to laboratory efficiencies. By 2017, 72% of labs had adopted modified or alternative ESR methods.7 Modified ESR, while having a shorter turn-around-time, still has many of the same environmental limitations as the Westergren method. Almost 1 in 3 testing facilities still rely on traditional techniques prone to environmental variability and limited sample stability. In 2025, there are a variety of automated ESR analyzers on the market that utilize photometric rheology, modified Westergren, and other alternate ESR methods (see Table 2).
The use of photometric rheology offers a compelling and easily adoptable solution to the required hands-on time and limited stability of Westergren and modified Westergren assays. These assays are correlated to the standard Westergren reference method but are engineered to improve laboratory efficiency by reducing hands-on time to seconds and turnaround time to minutes. Photometric rheology ESR analyzers are fully automated and less reliant on a technician’s skill and experience in performing the test. Therefore, there is less variability between users and shorter training times needed to successfully run the assay. The user-friendly automated workflow results in best-in-class precision and reproducibility between different operators and different labs. Longer sample stability — up to 28 hours at room temperature and 48 hours refrigerated — exceeds traditional recommendations for Westergren and modified Westergren assays. As lab testing becomes more consolidated and vacancy rates increase, greater flexibility in transport logistics and testing start times help maintain result quality. Additionally, photometric rheology ESR analyzers help reduce waste and lower risk of biohazard exposure. They utilize easily available capped EDTA tubes and not per-test disposables specific to older ESR test methods. Photometric rheology analyzers do not require any special sample preparation or uncapping of sample tubes; in addition, the no hands-on transfers of biological fluids help users avoid unnecessary biohazard exposure. These features of photometric rheology ESR analyzers can reduce manual workload and accelerate result delivery thereby addressing key pain points in today’s strained laboratory environments.
Conclusion
ESR tests are one of the most common routine lab tests, yet they still face considerable limitations. Photometric rheology represents a significant advancement in ESR testing. By minimizing environmental interference, reducing hands-on time and turnaround time, reducing user exposure to biological samples, and extending sample stability, it enhances laboratory efficiency and safety. As labs continue to face staffing shortages and increased testing demand, adopting such technologies may offer a practical path forward for ESR tests.
References
1. Watson J, Round A, Hamilton W. Raised inflammatory markers. BMJ. 2012;344(feb03 1):e454. doi:10.1136/bmj.e454.
2. Tishkowski K, Gupta V. Erythrocyte Sedimentation Rate. StatPearls Treasure Island (FL).
3. Reed L. Value of automated ESR. Medical Lab Management. Published online 2013:12.
4. Assasi N, Blackhouse G, Campbell K, et al. Comparative value of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) testing in combination versus individually for the diagnosis of undifferentiated patients with suspected inflammatory disease or serious infection: A systematic review and economic analysis [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015 Nov.
5. Grzybowski A, Sak J. A short history of the discovery of the erythrocyte sedimentation rate: LETTER TO THE EDITOR. Int J Lab Hematol. 2012;34(4):442-444. doi:10.1111/j.1751-553X.2012.01430.x
6. CLSI Document H02-A5. Clinical and Laboratory Standards Institute WVR, Suite 2500.; 2011.
7. Bull BS, Caswell M, Ernst E, et al. ICSH recommendations for modified and alternate methods measuring the erythrocyte sedimentation rate. J Clin Pathol. 1993;46(3):198-203. doi:10.1136/jcp.46.3.198.
8. Clinical laboratory personnel shortage. ASCLS. Accessed August 1, 2025. https://ascls.org/workforce/.
9. Forging a Path to Workplace Wellness: Insights From the Diagnostic Lab. QuidelOrtho. 2024.
10. 2025 NSI National Health Care Retention & RN Staffing Report. NSI Nursing Solutions, Inc. Accessed August 1, 2025. https://www.nsinursingsolutions.com/Documents/Library/NSI_National_Health_Care_Retention_Report.pdf.
11. Garcia E, Kundu I, Kelly M, Soles R. The American Society for Clinical Pathology 2022 Vacancy Survey of medical laboratories in the United States. Am J Clin Pathol. 2024;161(3):289-304. doi:10.1093/ajcp/aqad149.
About the Author

Richa Bedi, PhD, MHA, MS, MLT(ASCP)
is the Director of Laboratory Operations at Advocate Aurora Health in Chicago, Illinois. She ESRis responsible for laboratory leadership and spearheads initiatives to maintain compliance with accrediting bodies and quality improvement. Her role encompasses strategic planning, quality assurance, and managing laboratory operations to ensure the highest standards of patient care.

Carrie V. Vause, MS
is the Director of Content Development at the medical education company Medavera, Inc. She has over 17 years of experience in molecular research, education, and medical content development. Ms. Vause is published in peer-reviewed journals in cell and molecular biology and develops continuing education programs for healthcare professionals. Her focus is the creation of medical content that educates providers and impacts patient care.

Jane M. Caldwell, PhD
is the executive director of the medical education company Medavera, Inc. and has over 25 years of diverse experience with research and development in multiple areas of epidemiology and molecular biology. She has published extensively in peer-reviewed journals, book chapters, and the popular press. Dr. Caldwell recently worked as a consultant to troubleshoot algorithms for qPCR detection of COVID-19 infections which improved the accuracy of the kits filed under the Emergency Use Authorization.