How flow cytometers are making a difference in clinical labs
Flow cytometry was once relegated to sophisticated core laboratories, where only highly trained scientists could operate the complex instrumentation. Now, major advances in flow cytometry have made this technology more accessible and much easier to use — so easy, in fact, that more and more clinical laboratories have adopted flow cytometers for a wide variety of uses.
Even though many flow cytometers are considered “research use only” instruments, their compact footprint and reliable results have made them ideal for quality control steps that take place upstream of clinically regulated pipelines, as well as for laboratory-developed tests in CLIA-accredited facilities.
These platforms allow for extremely high-throughput analysis of cells or small particles. Thanks to the use of fluorescent labels, they can also be used to interrogate many different biomarkers or parameters at a time. Here, we’ll review some of the current and coming applications of flow cytometers in clinical labs, as well as key features to look for when choosing the right platform for your lab.
Sample assessment
Most clinical laboratories implement flow cytometers for quality control steps associated with ensuring sample viability or for purification before the sample is prepared for clinical testing. With their ability to stream thousands of cells per minute, flow cytometers are invaluable for handling any kind of QC process requiring counting, sorting, or basic analysis.
Cell counting, for example, is important for ensuring sufficient sample volume for some clinical assays. Assessing viability is also critical for many samples and is frequently needed for blood samples before any kind of test can be run on them. A purification step that pulls certain cells of interest from a broader population can also be accomplished with these instruments — for instance, separating specific immune cells in preparation for a clinical assay designed to detect natural killer cell activity.
These upstream steps are an excellent fit for RUO flow cytometers and can be performed with the simplest flow instruments available. Though these tasks are not technically part of running a clinical assay, they help to ensure that the downstream clinical results are as robust and reliable as possible.
In addition, flow cytometry may be implemented for clinical studies, where it can be used to monitor vaccine response by tracking antibody presence or to assess immune cell performance or patterns over time.
Flow features
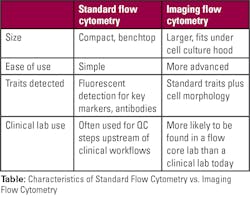
For lab personnel considering adopting a flow cytometer, there are a number of factors to consider. Different instruments have varying degrees of simplicity or complexity. If the tool will only be used for the basics — such as cell counting, sorting, and checking viability — then it makes sense to choose the system with best ease-of-use. More sophisticated technology should be reserved for situations where it’s really needed. Size is another key attribute; many compact systems are now available for situations where bench space is at a premium.
To ensure robustness and reproducibility, check not only system performance metrics but also the availability of software that includes straightforward modules or apps for each procedure. That reduces the need for training and increases the likelihood that the platform will run the same way every time.
Another option to consider is whether to adopt flow cytometry with imaging. This increases complexity as well as size, so it isn’t right for all laboratories. But platforms that pair flow cytometry with microscopy for image-based results can be powerful tools for more sophisticated sample analysis. These platforms make it possible to count and sort cells based on morphological differences in addition to biomarkers that can be tagged with fluorescent labels — a feature that can be useful with red blood cells and many other sample types.
Looking ahead
As more clinical laboratories adopt flow cytometers, their applications continue to expand. In the near future, flow cytometers may allow lab technicians to monitor a patient’s cancer by analyzing extracellular vesicles (EVs). There are already platforms available that have the resolution required to detect these vanishingly small particles and even to interrogate the protein or DNA cargo they may carry. While this application is primarily used in research labs right now, soaring interest in using EV analysis as a non-invasive cancer monitoring technique could lead to clinical adoption relatively soon.
Another near-term application for flow cytometers is micronuclei testing. Micronuclei populations in a cell are the result of exposure to toxic compounds, including radiation, and their analysis is likely to have strong clinical relevance. At the moment, identifying micronuclei is challenging, and doing so at the throughput needed is very difficult. Scientists are already working to develop this application with imaging-based flow cytometers.
As these and other protocols are developed for flow cytometers, what’s clear now is that these instruments are already quite useful in a clinical lab environment and appear likely to become even more important in the years to come
About the Author

Meredith Salisbury, BA
is a freelance journalist covering the life sciences field. She has extensive background reporting on genomics, molecular diagnostics, next-generation sequencing and a broad range of technologies.