Neutralization of red cell antibodies using soluble proteins: application to anti-CD38 interferences
Soluble substances with structures similar to blood group antigens have been used for decades in immunohematology testing to inhibit red cell antibodies with corresponding reactivity. This is a particularly useful technique to separate specificities in samples with multiple antibodies, to identify antibodies against a high prevalence antigen, or to eliminate reactivity attributed to a panreactive antibody, allowing for the identification of potential underlying non-neutralizable antibodies.
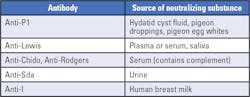
In this test, the neutralizing substance is first incubated with the serum, allowing the substance to bind to the variable regions of the target antibody. After incubation, the treated serum is then used to perform antibody identification. Several substances with blood-group antigen specificity are naturally present in body fluids, such as saliva, urine and plasma (Table 1)1. In addition, recombinant blood group proteins have been recently produced for other blood group antigens specificities, extending the potential application of the neutralization technique to a higher number of red blood cell antibodies.2
The resolution of drug-interferences during antibody identification is another area where the neutralization technique has been proven to be useful. Monoclonal antibodies represent a new and rapidly evolving class of immunotherapeutics used in the treatment of various conditions, such as solid tumors, leukemia and infections, as well as cardiovascular and inflammatory diseases.3
On November 16, 2015, the U.S. Food and Drug Administration (FDA) approved the first monoclonal antibody, daratumumab or DARA (anti-CD38), for the treatment of patients with multiple myeloma.5 Since then, several trials have been performed to extend the application of this new drug, or to develop other monoclonal antibodies with similar indications.6 During the phase I and II trial with DARA, the investigators observed some unexpected interferences in routine immunohematology testing. More specifically, all patients receiving DARA showed a positive indirect antiglobulin test (IAT), rendering difficult the identification of true red cell antibodies among these patients, and complicating the selection of suitable products for transfusion.7 These observations have been confirmed by numerous reports and are well-documented.8,9,10
Mitigating DARA interferences in immunohematology
Experts have proposed several methods to overcome the anti-CD38 interferences in immunohematology testing: chemical or enzymatic treatment of red cells; use of umbilical cord red cells for testing; extended phenotyping before or genotyping after initialization of DARA treatment; blocking of CD38 epitopes on the red cells using DARA-Fab fragments; and neutralization of DARA in patient’s serum using human soluble CD38 or mouse anti-DARA idiotype antibody.
Chemicals and enzymes
Among chemicals and enzymes used in routine immunohematology, dithiothreitol (DTT), papain and trypsin have been shown with different degrees of reproducibility to mitigate the interaction of DARA. DTT is a thiol-reducing agent that denatures red cell surface CD38 by disrupting the disulfide bonds in the molecule’s extracellular domain, therefore, preventing anti- CD38 from binding to the red cell.11 Papain and Trypsin are proteolytic enzymes that remove sialic acid residues on the red cell surface, modifying the structure of the corresponding proteins. By altering or cleaving the CD38 protein on the red cell, papain and trypsin have been shown to reduce interferences due to DARA treatment.12,13 The downside to using these chemicals and enzymes is the fact that, in addition to modifying CD38 expression on the red cell, they also denature other blood group antigens, impairing the identification of the corresponding antibodies in the patient’s serum (Table 2). Patients who have developed those specific antibodies are at high risk of a hemolytic transfusion, unless antigen negative blood is systematically provided to compensate for the effects of the enzyme treatment. This is particularly true for some DARA-patients who might develop anti-Kell antibodies after exposure to Kell antigens, or for who an existing anti-Kell may not be easily identified using DTT treated cells.
Umbilical cord red cells
Cord red cells have shown to be non-reactive with DARA. The interferences observed when testing adult red cells were not seen when using cord cells. Some laboratories have successfully established protocols using cord blood to mitigate the interferences observed in DARA patients.14 However, these techniques are not readily available in all laboratories because of the limited supply of cord cells. In addition, some red cells antigens are not expressed or only weakly present on cord cells,15 limiting their effectiveness to positively identify numerous clinically significant antibodies in DARA-patients.
Extended phenotyping or genotyping
Performing an extended blood-group phenotyping prior to initiating DARA treatment, or blood group genotyping after initiation of DARA, is an alternative strategy for dealing with the interferences seen during immunohematology testing.10 The rationale supporting this strategy is that extended phenotypically matched blood can be provided to the DARA-patient if needed, without the need for extensive additional testing. In order for this strategy to be efficiently implemented, there is a need for coordination between the oncologist in charge of the patient, the pharmacy, and the immunohematology laboratory. In addition, blood group genotyping needs to be accessible in a timely and cost-effective manner to guarantee optimal benefits for the patient.16 Also, according to current practices across laboratories, providing extended phenotypically matched blood does not eliminate the need for performing antibody screening or identification for transfused DARA-patients. Identifying suitable blood donors that are extendedly matched to the patient is also an issue, especially in DARA-patients needing multiple blood transfusions.
Blocking and neutralization
Blocking the DARA binding site by using an anti-CD38 idiotype antibody demonstrated some success in laboratory experimentation.7,17 However, this method has not been widely used because of the high cost for producing such highly DARA-specific antibodies and the difficulty optimizing a complex assay in routine immunohematology testing. The use of soluble substances – i.e. proteins – for neutralizing the reactivity of DARA is another method that was demonstrated in laboratory experiments. However, reagents available during these initial studies were not very concentrated and required larger volumes for neutralization, which posed a risk of diluting weakly reactive antibodies. Subsequently, a recombinant soluble CD38 (sCD38) was developed, allowing the use of smaller volumes of reagent for use for neutralization. With the development of a concentrated sCD38 reagent, and the fact that immunohematology laboratory technologists are familiar with neutralization techniques, it seemed logical to implement a CD38-neutralization method as an optimal approach to dealing with these interferences. CD38 neutralization uses a sCD38 reagent that, when incubated with the DARA-patient serum, will neutralize the reactivity of anti-CD38 present in the serum (Figure 1), allowing for the detection of any underlying antibodies.18
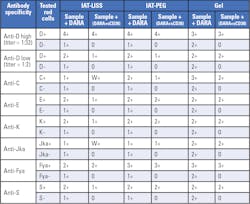
By testing the patient’s plasma with sCD38-treated plasma, the absence of reactivity observed in the IAT can be considered as a negative antibody screen. To develop the sCD38 neutralization assay, multiple examples of different red cell antibody specificities were spiked with DARA, to a final concentration of ~0.5mg/mL DARA. Recombinant soluble CD38 (sCD38) was then added to the sample and incubated for 30 minutes at room temperature. After inhibition, spiked samples were tested using IAT-LISS, IAT-PEG and Gel methods (Table 3).
Conclusion
Different strategies are available for mitigating the interference of daratumumab (anti-CD38) with immunohematology testing or providing alternative approaches for hemotherapy, each with its pros and cons. One of these strategies, neutralization, which is based on the principle of inhibition, and has been in use in blood bank laboratories for decades. This test effectively overcomes the CD38 interferences and allows for accurate identification of potential underlying alloantibodies against red cell antigens, which may be present in the patient’s plasma but masked by daratumumab interference. Additionally, the ruling out of underlying alloantibodies with a negative antibody screen with sCD38-treated plasma eliminates the need for an AHG crossmatch.
References:
- Byrne KM, Mercado C.M.C, Nnabue TN, Paige TD, Flegel WA. Inhibition of blood group antibodies by soluble substances. Immunohematology. 2019 Jan; 35(1): 19–22.
- Seltsam A, Wagner F, Lambert M, et al. Recombinant blood group proteins facilitate the detection of alloantibodies to high-prevalence antigens and reveal underlying antibodies: results of an international study. Transfusion. 2014; 54:1823–30. doi: 10.1111/trf.12553.
- Ruuls SR, Lammerts van Bueren JJ, van de Winkel JG, et al. Novel human antibody therapeutics: the age of the Umabs. Biotechnol J. 2008; 3:1157-71. doi: 10.1002/biot.200800110.
- Trudell KS. Detection and identification of antibodies. Modern Blood Banking & Transfusion Practices. Harmening D. F.A. Davis Company 2012; 6th Ed:216-240.
- Raedler LA. Darzalex (Daratumumab): First anti-CD38 monoclonal antibody approved for patients with relapsed multiple myeloma. Am Health Drug Benefits. 2016; 9(Spec Feature):70-3.
- Tong B, Wang M. CD47 is a novel potent immunotherapy target in human malignancies: current studies and future promises. Future Oncol. 2018;14(21):2179-2188. doi: 10.2217/fon-2018-0035.
- Oostendorp M, Lammerts van Bueren JJ, Doshi P, Khan I, Ahmadi T, Parren PWHI, van Solinge WW, De Vooght K.M.K. When blood transfusion medicine becomes complicated due to interference by monoclonal antibody therapy. Transfusion. 2015; 55(6 Pt 2):1555-62. doi: 10.1111/trf.13150.
- De Vooght KM, Oostendorp M, van Solinge WW. New mAb therapies in multiple myeloma: interference with blood transfusion compatibility testing. Curr Opin Hematol. 2016; 23(6):557-562. doi: 10.1097/MOH.0000000000000276.
- Murphy MF, Dumont LJ, Greinacher A; BEST Collaborative. Interference of new drugs with compatibility testing for blood transfusion. N Engl J Med. 2016;375(3):295-6. doi: 10.1056/NEJMc1515969.
- De Vooght KM, Oostendorp M, van Solinge WW. Dealing with anti-CD38 (daratumumab) interference in blood compatibility testing. Transfusion. 2016; 56(3):778-9. doi: 10.1111/trf.13474.
- Chapuy CI, Aguad MD., Nicholson RT., AuBuchon JP, Cohn CS, Delaney M., Fung MK, Unger M, Doshi P., Murphy MF, Dumont LJ, Kaufman RM, and the DARA-DTT Study Group for the BEST Collaborative. International validation of a dithiothreitol (DTT)-based method to resolve the daratumumab interference with blood compatibility testing. Transfusion 2016; 56: 2964-72. doi: 10.1111/trf.13789.
- Berthelier V, Laboureau J, Boulla G, Schuber F, Deterre P. Probing ligand induced conformational changes of human CD38. Eur J Biochem. 2000; 267:3056-64. doi: 10.1046/j.1432-1033.2000.01329.x.
- Carreño-Tarragona G, Cedena T, Montejano L, Alonso R, Miras F, Valeri A, Rivero AJ Lahuerta JJ, Martinez-Lopez J. Papain-treated panels are a simple method for the identification of alloantibodies in multiple myeloma patients treated with anti-CD38-based therapies. Transfus Med. 2019;29(3):193-196.
- Schmidt AE, Kirkley S, Patel N, Masel D, Bowen R, Blumberg N. An alternative method to dithiothreitol treatment for antibody screening in patients receiving daratumumab. Transfusion. 2015 Sep;55(9):2292-3. doi.org/10.1111/trf.13174
- Reid ME, Lomas-Francis C, Olsson ML. The Blood Group Antigen Factsbook. Cambridge, MA: Academic Press, Elsevier; 2012; 3rd Ed.
- Cushing MM, DeSimone RA, Goel R, Hsu YS, Parra P, Racine-Brzostek SE, Degtyaryova D, Lo DT, Morrison M, Crowley KM, Rossi A, Vasovic LV. The impact of daratumumab on transfusion service costs. Transfusion. 2019 Apr;59(4):1252-1258.
- Chapuy CI, Nicholson RT, Aguad MD, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion 2015(6Pt2); 55: 1545-54. doi: 10.1111/trf.13069.
- Binda M, Favaloro V, Jody D, Berry JD, Schwind P. Novel Soluble CD38 for Efficient Neutralization of High Titer Anti-CD38 Antibodies. Transfusion. 2018;58(S2):171A.
About the Author

Ghislain Noumsi MD, MBA, SBB
is the Director, Medical Affairs Asia-Pacific for Grifols Diagnostics Solutions Inc. Noumsi is an immunohematologist specialist with clinical and laboratory expertise in the resolution of complex cases in transfusion medicine, and proven track record In the laboratory pharmaceutical industry.
Lauro Guerra MLS, SBB
is a Laboratory Manager in Grifols Immunohematology Center, San Marcos, TX . Guerra is a medical laboratory technologist, with experience in immunohematology laboratory testing, using advanced pre-transfusion serological techniques