New HA and HB therapies offer innovation for patients and expanded testing opportunities for laboratories
In Plato’s dialogue Phaedrus, the titular protagonist states, “Things are not always what they seem; the first appearance deceives many; the intelligence of a few perceives what has been carefully hidden.”
Accordingly, how frustrated do you feel when you receive the wrong dish at a restaurant, or the wrong product after ordering online? More relatable to our everyday life in the lab—what happens if you expect one result after carefully running a lab test, but you observe a different result instead? Most would find it very frustrating and would want to understand the root cause.
New hemophilia therapies
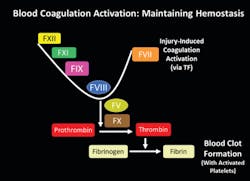
Presented here is valuable information around new therapies for patients with the rare, serious inherited bleeding disorders hemophilia A (HA) and hemophilia B (HB). Factor VIII (FVIII) and factor IX (FIX) are the coagulation factors missing in patients with HA and HB, respectively. Regular infusions are required to maintain balance in the coagulation system to ensure bleeding stops in response to injury (Figure 1). Though males are mostly affected, due to the X chromosome location of the genes encoding FVIII and FIX, women are also affected if they carry two mutated copies of the gene, or if they carry one copy but also have other risk factors for bleeding. Some of the currently available and new HA and HB therapies require intimate knowledge of the different patient therapy options, or patient care could suffer due to the unexpected effects of some therapies on commonly used coagulation assays.
The new therapies present tricky problems faced daily by medical technologists, especially those in reference labs, university hospitals, children’s hospitals, and other centers associated with hemophilia treatment centers (HTCs). Traditional clotting assays used in the lab, especially activated partial thromboplastin (aPTT) assays used for screening, along with a PTT-based one-stage clotting assays (OSAs) may show unexpectedly low or high results with certain current therapies.
The most common FVIII and FIX assays used in clinical laboratories are based on the OSA method. Chromogenic substrate assays (CSA) are also available, but may require extensive validation, as dictated by local practices. Though CSAs are generally less vulnerable to interference, CSAs are not a one-size fits all solution and may also experience interference from some therapies.
Changing landscape
The HA and HB therapy landscape is rapidly changing. The introduction of new therapies results in lab staff needing to understand each therapy and the effect on coagulation assays. If the lab is not kept abreast of new patient therapies in use, unexpected results may arise, causing confusion or potential patient care risks.
In the past, the only option for HA and HB patient treatment was plasma replacements, followed by introduction of more effective plasma-derived FVIII or FIX. Recombinant FVIII and FIX therapies were introduced to improve patient safety, but recent years have seen an explosion of new options for HA and HB patients designed to decrease the dosing frequency.
The newer generation extended half-life (EHL) therapies take several forms, including B-domain deleted (BDD) FVIII, or factor fusions to the Fc domain of human IgG1, albumin, or polyethylene glycol (PEG). Each modification strategy allows for reduction in the rate of clearance of the therapy from the body while reducing incidence of autoantibody, or inhibitor development. Thus, all of the aforementioned strategies allow for significant reductions in dose frequency and more effective treatment, allowing greatly improved quality of life.
Recent breakthroughs
A more recent breakthrough in HA therapy was the introduction of emicizumab (HEMLIBRA), a bispecific antibody recognizing both human activated factor IX (FIXa) and factor X (FX), bypassing the need for FVIII. Most importantly, emicizumab can be given subcutaneously, allowing patients to self-dose with greater ease compared to other therapies.
Further innovations are currently in progress for HA and HB patients, including antibodies targeting tissue factor pathway inhibitor (TFPI), silencing RNA (siRNA) targeting antithrombin (AT), and genetic therapies containing replacement FVIII and FIX. New therapies in development offer potentially more benefits for HA and HB patients, including subcutaneous dosing, reduced dosing frequencies, utilization of the same therapy for both HA and HB patients, and potentially curative outcomes for at least some patients.
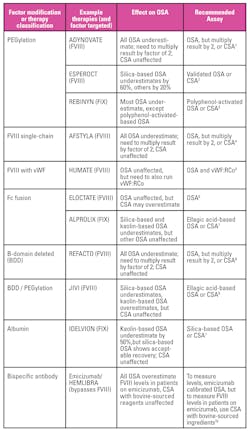
For the lab, some of the existing EHL therapies have produced confounding results. For example, PEGylated FVIII and FIX interfere with some OSA activators, due to the interactions of the PEG molecule with the aPTT activator surface (Table 1). Other factor modifications described here may also show different effects from FVIII and FIX molecules with no modifications. Due to interference issues, labs may choose to use a chromogenic substrate assay (CSA) for monitoring patients on selected EHL therapies, but if the clinicians do not communicate with the lab regarding the specific patient therapy in use, the lab will not know whether to choose the OSA or CSA. In addition, some of the therapies, including BDD FVIII require the OSA result to be multiplied by two, regardless of the OSA activator, and it is unclear whether the lab would need to validate the multiplication factor, or the clinician simply interprets the OSA result for the selected patients.
With the introduction of emicizumab, which does not require routine drug or FVIII level monitoring, the lab may still need to monitor FVIII levels in patients experiencing breakthrough bleeding receiving regular FVIII infusions. If FVIII levels are needed, no OSA can accurately monitor FVIII levels unless an emicizumab calibrator and control are used. Alternatively, a CSA may be used for the emicizumab patients receiving FVIII infusions, but if the patient has a FVIII auto-antibody, or FVIII inhibitor, then only a CSA with bovine-sourced reagents can be used. On the other hand, if the patient does not have a FVIII inhibitor, and clinicians want to measure emicizumab drug levels, then only a CSA with human-sourced reagents can be used, or an emicizumab-calibrated OSA. Thus, care of emicizumab patients requires clinicians and labs to closely work together on patient monitoring practices.
Use of a CSA may be justified by labs for more than just EHL therapy monitoring. CSA approaches are useful for patients with lupus anticoagulants affecting the OSA, and also for patients with certain FVIII and FIX gene mutations associated with non-severe HA and HB.10 In the case of the latter two situations, CSA will best allow for correct diagnosis and treatment monitoring.
Conclusion
In conclusion, specialized clinical labs, especially those associated with HTCs will have patients on many different HA and HB treatments, both standard and EHL, potentially requiring them to utilize OSA with different activators, along with CSAs. Generally, it will not be possible to only use one OSA or one CSA method for all patients requiring FVIII and FIX testing. As laboratorians following the words of Plato, we must always be prepared to investigate what lies behind the facade. Armed with the correct information, we are in the best position to find the reasons for unexpected results and ensure the best possible patient care is provided safely.
REFERENCES
Pruthi R.K., Laboratory monitoring of new hemostatic agents for hemophilia. Semin Hematol, 2016. 53(1): 28-34.About the Author

Paul Riley, PhD, MBA
serves as Scientific Business Development Manager at Diagnostica Stago, Inc. Paul earned a PhD in biochemistry from Temple University in 2006, with the subject of the dissertation regarding the function of coagulation factor XI. He also did postdoctoral training in a related area before becoming a product manager and scientific affairs specialist in 2009. During his time at Stago, Paul also completed an MBA degree program at Cornell University in 2014. He has spoken to dozens of medical technologist, clinical laboratory scientist, pharmacist, and clinician audiences about various topics within hemostasis and coagulation.