Cancer biomarker types and clinical applications
To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
1. Define how cancer biomarkers are used and list their types or biomolecules.
2. Discuss how genetic and epigenetic biomarkers are used and the latest emerging tests in this category.
3. Discuss the advantages and disadvantages of protein biomarkers and the mechanisms of action related to testing for them.
4. Discuss the mechanisms and types of metabolic biomarker testing to aid in cancer care.
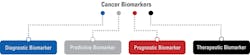
The National Cancer Institute defines a biomarker as a biological molecule found in blood, other body fluids, or tissues that indicates if a process, or a condition or a disease such as cancer is normal or abnormal. A biomarker is also called a molecular marker or signature molecule.1 Cancer biomarkers are classified in different ways based on their use (See Figure 1).
Cancer biomarkers are used to:3,4
- Assess patients in multiple clinical settings to identify, estimate risk of disease, screen for occult primary cancers, distinguish benign from malignant type, characterize one type of malignancy from another type (for example, BRCA1 germline mutation for breast and ovarian cancer5 and Prostate specific antigen (PSA) for prostate cancer6)
- Determine prognosis and predict chance of survival or recurrence for patients who have been diagnosed with cancer (for example, 21 gene in Oncotype Dx for breast cancer8)
- Monitor status of the disease
- Inform individualized treatment plans (for example, immunohistochemistry for various cancers7 and KRAS mutations in Exon 2, 3 and 4 for colorectal cancer9)
- Detect recurrence or determine response or progression to therapy (for example, 22 gene in Decipher Prostate10 and cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) for breast cancer11)
- Anticipate and manage negative medication reactions
Cancer biomarkers based on the type of biomolecules
Cancer biomarkers can be broadly categorized into their biological nature — genetic, epigenetic, transcriptomic, proteomic, and metabolic.3
Genetic biomarkers
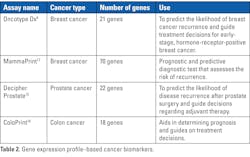
Genetic biomarkers for cancer are specific genes, gene mutations, or gene expression patterns that can indicate the presence and stage of cancer or the potential for cancer development. Gene mutations and gene alterations can provide valuable information about the underlying genetic changes driving the development and progression of cancer. Table 1 provides a few examples of gene mutation– and gene alteration–based cancer biomarkers. Gene expression profiles provide insights into tumor behavior, prognosis, and treatment response. Table 2 provides a few examples of gene expression profile–based cancer biomarkers.
DNA as a cancer biomarker
Cancer cells release nucleic acids, freely or associated with other structures such as vesicles into body fluids, including blood. Among these nucleic acids, circulating tumor DNA (ctDNA) has emerged as a minimally invasive biomarker for cancer.19 Circulating tumor DNA (ctDNA) has now emerged as a very promising noninvasive biomarker for cancer diagnosis, prognosis, and therapeutic monitoring, providing significant potential for real-time insights into tumor dynamics.20 The circulating tumor DNA (ctDNA) is a key component of liquid biopsy tests. Table 3 provides examples of a few FDA-approved liquid biopsy ctDNA tests.
RNA as a cancer biomarker
RNA molecules, including messenger RNA (mRNA), microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA), are emerging as promising cancer biomarkers due to their dynamic nature and potential for non-invasive detection in bodily fluids. Their levels can indicate the presence, stage, and progression of various cancers, providing valuable information for early diagnosis, prognosis, and treatment monitoring. Like circulating tumor DNA, RNAs are also detected in liquid biopsy tests. That said, RNA-based liquid biopsy tests are not yet that prevalent as DNA-based liquid biopsy tests. Researchers at the University of Chicago for the first time used RNA modifications to develop a liquid biopsy test for colorectal cancer that was more sensitive than DNA-based liquid biopsy test.24
Epigenetics as a cancer biomarker
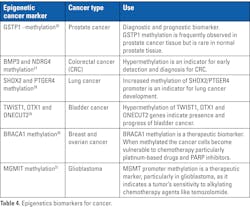
Epigenetic changes including DNA methylation, histone modifications, nucleosome positioning, and non-coding RNAs, particularly microRNAs are essential for normal gene expression and proper cellular functioning. Any disruption of this mechanism results in a change in gene function and eventually development of cancer. Hence detection of aberrant epigenetic patterns can serve as biomarkers for early detection, prognosis, and potential targets for therapeutic intervention.25 Table 4 provides examples of a few epigenetics biomarkers for cancer.
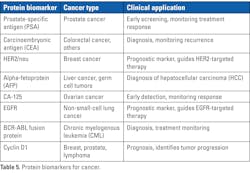
Protein as cancer biomarker
Protein biomarkers are specific proteins present in biological fluids (such as blood, urine, or saliva) or tissues that provide valuable information about the presence and progression of cancer.32 A majority of the tumor markers are either protein or peptides. Tumor biomarkers have revolutionized cancer diagnostics as they are non-invasive and can be used for the following:33
- Screening and early detection of cancer
- Aid in the diagnosis of cancer
- Determine response to therapy
- Prognostic indicator of disease progression
- Indicate relapse during follow-up period
That said, tumor markers also have disadvantages as below:
- They have very low concentrations or may not be present for early-stage cancer.
- Not standardized as proteins and/or modified proteins; may vary among individuals, between cell types, and even within the same cell under different stimuli or different disease states.
- Tumor markers may be present even in noncancerous conditions, hence not very specific for cancer.
The different types of protein biomarkers are as follows:
- Oncofetal antigens
- Tumor-associated antigens
- Hormones and hormone receptors
- Enzymes and isoenzymes
- Serum and tissue proteins
- Cancer stem cells
Mechanisms behind cancer-related protein biomarkers
Cancer cells undergo several molecular and genetic changes that result in the overproduction, alteration, or loss of normal proteins. These changes result in new proteins or modified versions of existing proteins that are released into the bloodstream or other bodily fluids, which act as diagnostic or prognostic biomarkers. Some key mechanisms include the following:
- Overexpression of growth factors: Cancer cells often overproduce growth factors, leading to uncontrolled cell proliferation.
- Loss of tumor suppressor proteins: Tumor suppressor proteins, such as p53, are often mutated or down regulated in cancer cells, contributing to uncontrolled cell growth.
- Altered post-translational modifications: Changes in protein modifications such as phosphorylation, glycosylation, or cleavage can produce altered protein isoforms detectable in blood samples. Table 5 provides examples of a few protein biomarkers for cancer.32
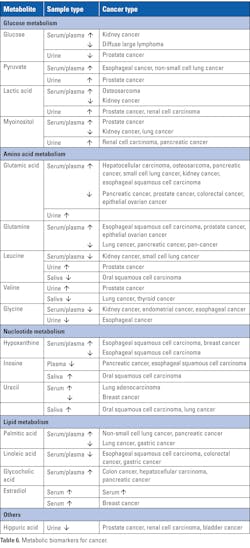
Metabolic biomarkers
Cancer has an effect on metabolic pathways and causes alterations in metabolites resulting in inappropriate proliferation of cancer cells and adaptation to the tumor microenvironment. The aberrant metabolites play pivotal roles in tumor formation and metastasis and thus serve as potential biomarkers for personalized cancer therapy.34 Table 6 provides a few examples of metabolic biomarkers for cancer.35
Due to the risk of false positive or negative results, it is advisable to combine a test for a cancer biomarker with another method such as tissue biopsy or endoscopy to improve the effectiveness of screening for cancer.36,37 A study showed that the combined detection of alpha-fetoprotein (AFP) with cell-free DNA (cfDNA) can improve the specificity of hepatocellular carcinoma (HCC) diagnosis to 94.4%, which was superior to that of AFP alone in terms of higher sensitivity and better clinical correlation.38
Methods of detection of cancer biomarkers
The traditional method for detection of cancer has been the detection of tissue biopsy and cytology to examine tissue and imaging techniques like positron emission tomography scan (PET Scan), computed tomography scan (CT Scan) and magnetic resonance imaging (MRI) to assess tumors. However, the various cancer biomarkers can be detected using a variety of methods, including immunoassays (like ELISA) for proteins; molecular techniques such as polymerase chain reaction (PCR), real-time quantitative PCR (qRT-PCR), digital PCR (dPCR), microarrays, and next-generation sequencing (NGS) for genetic alterations; mass spectrometry to identify proteins and metabolites; and advanced biosensors and nanomaterial-based methods for rapid, highly sensitive detection. The non-invasive liquid biopsy technique that detects circulating tumor cells (CTC), cell-free DNA (cfDNA) extracellular vesicles (EV), or circulating tumor DNA (ctDNA) in body fluids has also become very promising in detecting cancer biomarkers.
Conclusion
Despite the emergence of various newer techniques and technological advancements, on average, 1,700 deaths occur daily from cancer in the United States.39 Hence, the fight against cancer is still not over. Artificial intelligence (AI) has revolutionized various fields including healthcare, and it can help to reshape how we understand, diagnose, and treat patients. By using emerging multi-omics technology in combination with AI, we hope to provide personalized treatment to cancer patients and save lives in ways that were never possible before.
References
- Definition of biomarker- NCI Dictionary of Cancer Terms. National Cancer Institute. Accessed August 26, 2025. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomarker.
- Carlomagno N, Incollingo P, Tammaro V, et al. Diagnostic, predictive, prognostic, and therapeutic molecular biomarkers in third millennium: A breakthrough in gastric cancer. Biomed Res Int. 2017;2017:7869802. doi:10.1155/2017/7869802.
- Das S, Dey MK, Devireddy R, Gartia MR. Biomarkers in cancer detection, diagnosis, and prognosis. Sensors (Basel). 2023;24(1):37. doi:10.3390/s24010037.
- Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol. 2012;6(2):140-6. doi:10.1016/j.molonc.2012.01.010.
- Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56(1):265-71.
- Lin K, Lipsitz R, Miller T, Janakiraman S; U.S. Preventive Services Task Force. Benefits and harms of prostate-specific antigen screening for prostate cancer: An evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(3):192-9. doi:10.7326/0003-4819-149-3-200808050-00009.
- Duraiyan J, Govindarajan R, Kaliyappan K, Palanisamy M. Applications of immunohistochemistry. J Pharm Bioallied Sci. 2012;4(Suppl 2):S307-9. doi:10.4103/0975-7406.100281.
- Bernhardt SM, Dasari P, Wrin J, et al. Discordance in 21-gene recurrence scores between paired breast cancer samples is inversely associated with patient age. Breast Cancer Res. 2020;22(1):90. doi:10.1186/s13058-020-01327-1.
- Zenonos K, Kyprianou K. RAS signaling pathways, mutations and their role in colorectal cancer. World J Gastrointest Oncol. 2013;5(5):97-101. doi:10.4251/wjgo.v5.i5.97.
- Decipher Prostate Genomic Classifier. Veracyte. Accessed August 26, 2025. https://www.veracyte.com/decipher-prostate/.
- Hasan D. Diagnostic impact of CEA and CA 15-3 on chemotherapy monitoring of breast cancer patients. J Circ Biomark. 2022;11:57-63. doi:10.33393/jcb.2022.2446.
- Tangella LP, Clark ME, Gray ES. Resistance mechanisms to targeted therapy in BRAF-mutant melanoma - A mini review. Biochim Biophys Acta Gen Subj. 2021;1865(1):129736. doi:10.1016/j.bbagen.2020.129736.
- Passaro A, Mok T, Peters S, et al. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J Thorac Oncol. 2021;16(5):764-773. doi:10.1016/j.jtho.2020.12.002.
- Pujol P, Barberis M, Beer P, et al. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur J Cancer. 2021;146:30-47. doi:10.1016/j.ejca.2020.12.023.
- Amisha F, Malik P, Saluja P, et al. A comprehensive review on the role of human epidermal growth factor receptor 2 (HER2) as a biomarker in extra-mammary and extra-gastric cancers. Onco (Basel). 2023;3(2):96-124. doi:10.3390/onco3020008.
- Ma R, de Pennington N, Hofer M, Blesing C, Stacey R. Diagnostic and prognostic markers in gliomas - an update. Br J Neurosurg. 2013;27(3):311-5. doi:10.3109/02688697.2012.752432.
- MammaPrint – Breast Cancer Testing. Agendia. Accessed August 26, 2025. https://agendia.com/mammaprint/.
- Agendia announces launch of ColoPrint for colon cancer prognosis and prediction. Agendia. June 1, 2012. Accessed August 26, 2025. https://agendia.com/agendia-announces-launch-of-coloprint-for-colon-cancer-prognosis-and-prediction/.
- Sánchez-Herrero E, Serna-Blasco R, Robado de Lope L, et al. Circulating tumor DNA as a cancer biomarker: An overview of biological features and factors that may impact on ctDNA analysis. Front Oncol. 2022;12:943253. doi:10.3389/fonc.2022.943253.
- Asnaghi R, Marsicano RM, Fuorivia V, et al. Circulating tumor DNA: a biomarker for oncology drug development in phase I clinical trials? Expert Rev Mol Diagn. Published online 2025:1-9. doi:10.1080/14737159.2025.2531065.
- Torres S, González Á, Cunquero Tomas AJ, et al. A profile on cobas EGFR Mutation Test v2 as companion diagnostic for first-line treatment of patients with non-small cell lung cancer. Expert Rev Mol Diagn. 2020;20(6):575-582. doi:10.1080/14737159.2020.1724094.
- Jatkoe T, Wang S, Odegaard JI, et al. Clinical validation of companion diagnostics for the selection of patients with non-small cell lung cancer tumors harboring epidermal growth factor receptor exon 20 insertion mutations for treatment with amivantamab. J Mol Diagn. 2022;24(11):1181-1188. doi:10.1016/j.jmoldx.2022.07.003.
- FoundationOne Liquid CDX Technical Specifications. Foundation Medicine. March 2025. Accessed August 26, 2025. https://www.foundationmedicine.com/sites/default/files/media/documents/2025-06/F1LCDx_Tech%20Specs_SPEC-01746_R4.pdf.
- Ju CW, Lyu R, Li H, et al. Modifications of microbiome-derived cell-free RNA in plasma discriminates colorectal cancer samples. Nat Biotechnol. Published online 2025:1-7. doi:10.1038/s41587-025-02731-8.
- Tiwari N, Mishra J, Singh N, et al. Epigenetics and cancer stem cells-The world of cancer genesis: A review. J Cancer Res Ther. 2025;21(1):5-9. doi:10.4103/jcrt.jcrt_388_24.
- Ye J, Wu M, He L, et al. Glutathione-S-transferase p1 gene promoter methylation in cell-free DNA as a diagnostic and prognostic tool for prostate cancer: A systematic review and meta-analysis. Int J Endocrinol. 2023;2023:7279243. doi:10.1155/2023/7279243.
- Müller D, Győrffy B. DNA methylation-based diagnostic, prognostic, and predictive biomarkers in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2022;1877(3):188722. doi:10.1016/j.bbcan.2022.188722.
- Weiss G, Schlegel A, Kottwitz D, König T, Tetzner R. Validation of the SHOX2/PTGER4 DNA methylation marker panel for plasma-based discrimination between patients with malignant and nonmalignant lung disease. J Thorac Oncol. 2017;12(1):77-84. doi:10.1016/j.jtho.2016.08.123.
- van Kessel KE, Van Neste L, Lurkin I, Zwarthoff EC, Van Criekinge W. Evaluation of an epigenetic profile for the detection of bladder cancer in patients with hematuria. J Urol. 2016;195(3):601-7. doi:10.1016/j.juro.2015.08.085.
- Stefansson OA, Hilmarsdottir H, Olafsdottir K, et al. BRCA1 promoter methylation status in 1031 primary breast cancers predicts favorable outcomes following chemotherapy. JNCI Cancer Spectr. 2019;4(2):pkz100. doi:10.1093/jncics/pkz100.
- Butler M, Pongor L, Su YT, et al. MGMT status as a clinical biomarker in glioblastoma. Trends Cancer. 2020;6(5):380-391. doi:10.1016/j.trecan.2020.02.010.
- Damodar SV, Shukla VK, Kumar V, Deshmukh MV. The importance of protein biomarkers in cancer detection - the use of specific proteins as biomarkers for early cancer diagnosis and prognosis. doi:10.53555/AJBR.v28i1S.6406.
- Nagpal M, Singh S, Singh P, Chauhan P, Zaidi MA. Tumor markers: A diagnostic tool. Natl J Maxillofac Surg. 2016;7(1):17-20. doi:10.4103/0975-5950.196135.
- Wang W, Rong Z, Wang G, Hou Y, Yang F, Qiu M. Cancer metabolites: promising biomarkers for cancer liquid biopsy. Biomark Res. 2023;11(1):66. doi:10.1186/s40364-023-00507-3.
- Wang W, Zhen S, Ping Y, Wang L, Zhang Y. Metabolomic biomarkers in liquid biopsy: accurate cancer diagnosis and prognosis monitoring. Front Oncol. 2024;14:1331215. doi:10.3389/fonc.2024.1331215.
- Calabrese F, Lunardi F, Pezzuto F, et al. Are there new biomarkers in tissue and liquid biopsies for the early detection of non-small cell lung cancer? J Clin Med. 2019;8(3):414. doi:10.3390/jcm8030414.
- Bresalier RS, Grady WM, Markowitz SD, et al. Biomarkers for early detection of colorectal cancer: The early detection research network, a framework for clinical translation. Cancer Epidemiol Biomarkers Prev. 2020;29(12):2431-2440. doi:10.1158/1055-9965.EPI-20-0234.
- Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019;18(1):114. doi:10.1186/s12943-019-1043-x.
- Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871.
To take the test online go HERE. For more information, visit the Continuing Education tab.
About the Author

Rajasri Chandra, MS, MBA
is a global marketing leader with expertise in managing upstream, downstream, strategic, tactical, traditional, and digital marketing in biotech, in vitro diagnostics, life sciences, and pharmaceutical industries. Raj is an orchestrator of go-to-market strategies driving complete product life cycle from ideation to commercialization.