Despite being a global healthcare issue that kills more people than cancer, sepsis is still not widely known. Only about half of Americans have heard of sepsis,1 although 258,000 people die from sepsis every year in the United States. That is one every two minutes, more than from prostate cancer, breast cancer, and AIDS combined.2 Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.3 In effect, the body’s response to combat infection causes organ damage and failure, which in some people leads to death within hours or results in long-lasting effects, if not treated in a timely fashion.
An estimated 30 million people worldwide are affected by sepsis each year.4 In the U.S. alone, the number of hospital admissions for sepsis has increased up to three-fold over the last decade. In the same period, hospital admissions for stroke and myocardial infarction remained stable.5
Diagnosing sepsis has traditionally relied on clinical signs followed by laboratory-based tests, such as blood cultures, which may allow the identification of the pathogen. Unfortunately, all laboratory tests have turnaround times of hours or even days. It is vital that antimicrobial treatment be promptly started in patients who need it. Delays in initiating antimicrobial treatment are correlated with a progressive increase in mortality due to severe sepsis and septic shock.6 However, the rising global problem of antimicrobial resistance imposes a pragmatic use of antibiotics, where doctors prescribe antibiotics empirically.7
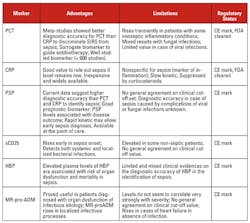
Early recognition of sepsis and monitoring treatment efficacy are key to address this global crisis. Currently available biomarkers for sepsis include procalcitonin (PCT) and C-reactive protein (CRP). Neither biomarker, however, is sufficiently specific or sensitive to identify sepsis patients in a timely manner.
A sepsis biomarker that can be quantitatively and rapidly measured at the point-of-care is urgently needed to discriminate non-infectious from infectious inflammation, providing information that enables physicians to make the most appropriate medical decision. The combination of novel, rapid IVD platforms and biomarkers with high diagnostic accuracy is currently in development.
Further defining sepsis
Sepsis is a life-threatening condition caused when the body’s response to an infection injures its own tissues and organs.8 Patients with suspected infection are likely to have a prolonged stay in intensive care or die in the hospital. At the bedside, protocols are available to aid clinicians in identifying sepsis patients, such as the qSOFA (quick sepsis-related organ failure assessment), which monitors alteration in mental status, systolic blood pressure ≥100 mm Hg, or respiratory rate ≥22/min. Patients meeting two or more of these criteria are deemed at a greater risk for a poor outcome.
However, sepsis is a very heterogenous disease, and clinical phenotypes of a patient may be similar to that of sterile, systemic inflammation. Severe symptoms in sepsis can develop very quickly. High-risk patients need to be identified and treated promptly to avoid debilitating problems such as heart failure or limb amputation, and, in the worst cases, death. Recent treatment guidelines emphasize the need for these patients to be treated with antibiotics and IV fluids within an hour.8 With the alarming rise in antimicrobial resistance and the emergence of multi-resistant bacteria in recent years, prophylactic antimicrobial treatment is problematic. Misuse of antimicrobials in patients where they are not needed only contributes to resistance.
The burden and the need
The incidence of sepsis is increasing rapidly. The U.S. Centers for Disease Control and Prevention’s National Center for Health Statistics estimates that the number of times people were hospitalized with sepsis increased from 621,000 in 2000 to 1,141,000 in 2008.9 Deaths from sepsis in the U.S increased from 154,159 in 2000 to 207,427 in 2007.10
Although many sepsis patients recover and return to normal life, some remain at increased risk for death in the following months and years, or go on to develop disabilities, both physical and mental.
Sepsis is the most expensive condition treated in U.S. hospitals, costing more than $20 billion in 2011, and increasing on average annually by 11.9 percent, according to The Agency for Healthcare Research and Quality.11 If sepsis were identified and treated earlier, it has been estimated that there would be 92,000 fewer deaths annually, 1.25 million fewer hospital days annually, and reductions in hospital expenditures of over $1.5 billion.12
As noted, comprehensive sepsis diagnosis today is delayed by the long turnaround time required for the quantitative measurement of blood proteins present at low concentration or the identification of pathogens. There are currently no rapid diagnostic tests available to accurately identify patients with sepsis. Therefore, there is a significant need to combine a rapid, near-patient diagnostic platform with the accurate measurement of proteins in complex matrices to improve sepsis patient outcome and optimize resources in ICU. This need was very recently highlighted in the World Health Organization’s (WHO) resolution on sepsis, which recognizes sepsis as a global health priority and supports the development and use of an innovative diagnostic approach for a better, (and earlier) diagnosis of sepsis.13
Emerging sepsis biomarkers
Recently, novel protein biomarkers have been validated with substantial clinical evidence in the identification of severe infection and/or sepsis and septic shock. (Other approaches, typically based on the measurement of gene expression profiling or molecular strategies to improve pathogen detection rather than sepsis itself, are not discussed here, although they also represent interesting developments.)
The two most commonly used blood protein biomarkers for diagnosing infections that may lead to sepsis are C-reactive protein (CRP) and procalcitonin (PCT). CRP is not sufficiently specific for diagnosing sepsis, as it can also become elevated during inflammatory states of non-infectious etiology, such as trauma, burns, cancer, cardiovascular disease, surgery, and autoimmune disease. PCT is a biomarker whose levels increase precipitously in patients with severe bacterial infection. It elevates prior to rises in lactic acid and is more specific for sepsis. Although an important aid in the diagnosis of sepsis, testing for PCT often takes hours to obtain a result.
Pancreatic stone protein (PSP)
One very promising new biomarker for sepsis that is emerging is pancreatic stone protein (PSP). PSP is mostly secreted by the acinar cells of the pancreas. Its exact physiological role is only partially understood, but it has been shown to activate neutrophil granulocytes.14
PSP has been evaluated in multiple clinical situations and has systematically been shown to be superior or similar to PCT. In particular, when used at the time of a patient’s admission to intensive or high dependency care, PSP was superior to PCT in differentiating sepsis from non-infective illnesses.15
If measured within 24 hours of ICU admission, PSP predicts risk of in-hospital death more accurately than both PCT and CRP. Interestingly, there is a linear relationship between PSP value and the probability of death.16 A later study conducted by Que et al17 confirmed the prognostic value of PSP in patients with severe infections requiring ICU management. The researchers developed and validated an in-hospital model that showed that the addition of PSP to clinical scores, such as APACH II and SAPS2, is superior in predicting mortality than the addition of PCT. Consequently, PSP could be a good biomarker to stratify patients admitted to the ICU.
A recently published study compared the role played by PSP, sCD25, and PCT in identifying infection and sepsis in the emergency department setting. PSP was found to be the best biomarker for the identification of sepsis, while PCT was found to be slightly better than PSP for infection.18
Post-operative sepsis occurs for about one third of sepsis cases worldwide. PSP was evaluated in post-cardiac surgery for its diagnostic accuracy for complications of infectious origin and found to be superior to CRP and white blood cell count at identifying post-operative infection. The study also suggests that daily measurement of PSP after surgery may aid in early identification of infection.19
Clinical evidence demonstrated by these studies highlights the potential of the PSP biomarker to aid clinicians in the identification of patients with, or at risk of, severe infection and sepsis. Of particular note, the PSP level remains low in non-infectious SIRS, and its kinetics in infected and sepsis patients appears to evolve rapidly. That may open the door to a promising use of this biomarker to closely follow up patients identified as at risk of developing sepsis in order to fine-tune therapy, and possibly avoid misuse or overuse of antibiotics. Moreover, PSP’s correlation between blood level and outcomes also supports an interesting potential in risk stratification at triage.
Soluble CD14 (presepsin)
Soluble CD14 (sCD14, also named presepsin) is a fragment of the receptor for lipopolysaccharide (LPS). Its physiological role and mechanism of production in sepsis are unknown. A correlation of sCD14 levels and severity of disease (SIRS, sepsis, septic shock) has been shown, and multiple comparisons have highlighted an average diagnostic performance that is very similar to that of PCT. From all these studies, measurement of sCD14 may be useful in several clinical situations. As highlighted in a recent meta-analysis, no clear cut-off for clinical use has however been determined so far, with the authors suggesting that sCD14 only be used as part of a clinical assessment of sepsis rather than a standalone biomarker.20
MR-pro-adrenomedullin
Adrenomedullin level is modulated by the complement system. It is a potent vasodilator, with bactericidal effects. Assays measuring its mid-regional precursor fragment (MR-pro-ADM) have shown elevated levels in plasma from sepsis patients.
MR-pro-ADM is not per se a biomarker of a body inflammatory response to an infection, as is the case with PCT. However, MR-pro-ADM may aid in better prognosticating risk of mortality and severe complications. In community-acquired pneumonia (CAP) patients, a meta-analysis demonstrated that MR-pro-ADM is predictive of increasing complications in patients suffering from CAP. But, again, data to determine an optimal cut-off-level and potential benefit of combining this marker with severity scores or other biomarkers are still deemed insufficient.21
Heparin-binding protein
Heparin-binding protein (HBP) is a glycoprotein synthetized in neutrophils and released in the bloodstream upon activation. Its various roles contribute to the maintenance and progression of inflammation. Published data suggest that HBP may be useful in identifying patients who are at risk of developing sepsis with circulatory failure. It may also have a good diagnostic accuracy for the assessment of severe sepsis. Its clinical utility, however, is still difficult to assess, as mixed results were obtained in different clinical studies.22
Looking ahead
Sepsis etiology, clinical manifestations, and underlying molecular and cellular mechanisms are complex and heterogenous. Consequently, it is of utmost importance to foster development of tools to aid clinicians in the rapid identification of sepsis and risk stratification of patients at risk of severe infection and sepsis, and in the monitoring of disease progression and management of treatment, typically with antibiotics.
Decentralized testing is often seen as a challenge by laboratory managers. But technologies today enable high-quality, simple tests at the point of care. Blood gas analyzers have been in the ICU worldwide for decades. However, implementation of new point-of-care testing (POCT) in hospitals is not without challenges:
- Many people need to receive training, especially in large units. A test is needed with minimal steps and easy, intuitive handling and very low risk of misuse or misinterpretation of results, as tests are often performed by clinical staff rather than laboratory professionals.
- Quality control must be at best integrated, or regularly performed, and data/non-conform results must be immediately available for Quality Manager review (via integration of tests
results with HIS/LIS). Also, maintenance must minimal. - Advantages must overcome challenges; cost benefit must be clear, as well as patients’ benefit from more rapidly available test results.
Today’s technology allows the development and implementation of high-quality POCT for sepsis. The technical challenges highlighted for other microfluidic-based devices can be overcome by the development of nanofluidic-based testing, in which there are minimal pre-analytical steps, no maintenance is needed, and turnaround time is extremely short thanks to enhanced molecular interactions.23
It is important that clinical programs that evaluate new POCTs are designed and conducted in a way that clinicians can precisely know which biomarker measurement will bring a valuable benefit to their routine activities. Clinical programs for biomarker tests should focus on impact studies to definitely evaluate the efficacy of the potential biomarker in the daily activities of ED and ICU, on patient populations that match the reality of those units. Parameters such as cost-benefit, implementation of the device at the POC in ICU and ED (triage), acceptance by users, compatibility with laboratory requirements, and quality assurance should be considered in evaluating the use and potential of new biomarkers.
A rational use of biomarker testing may contribute to optimizing resources thanks to better triage and risk stratification. Such use can also help to optimize the administration of antimicrobials thanks to a better identification of patients who need them (discrimination of non-infectious from infectious SIRS, more or less likely to evolve toward sepsis and septic shock) and monitoring of treatment efficacy. Finally, a better understanding of the mechanisms of sepsis at the molecular, cellular, and organ level may aid in the optimal use of current and future sepsis biomarkers. Sepsis is not only about treating an infection; it is also about bringing appropriate support to patients with multiple organ dysfunctions. In that sense, molecular biology techniques identifying pathogens and biomarkers of inflammation and organ dysfunction must be readily available to clinicians to enable them to make the best medical decisions.
REFERENCES
- Sepsis Awareness Research 2016, http://sepsis.org/files/sasepsisawareness2016.pdf.
- Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. Hospitals, 2009: Statistical Brief #122. 2011 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb. https://www.ncbi.nlm.nih.gov/books/NBK65391/.
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
- Global Sepsis Alliance, http://global-sepsis-alliance.org/global-burden/.
- Seymour CW, Rea TD, Kahn JM, Walkey AJ, Yealy DM, Angus DC. Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186(12):1264-1271.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596.
- Tackling Drug-Resistant Infections Globally: Final report and recommendations, May 2016 https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- NICE Sepsis Guidelines, March 2017, https://www.nice.org.uk/guidance/indevelopment/gid-qs10032.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A: Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. In: NCHS data brief Hyattsville, MD: National Center for Health Statistics. vol. 62;2011.
- Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK: Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012; 40(3):754-761.
- Torio CM, Andrews RM: National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011: Statistical Brief #160. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville (MD): Agency for Health Care Policy and Research (US) 2006-2013.
- Shorr AF, Micek ST, Jackson WL, Jr., Kollef MH: Economic implications of an evidence-based sepsis protocol: can we improve outcomes and lower costs? Crit Care Med. 2007;35(5):1257-1262.
- World Health Organization. Improving the prevention, diagnosis and management of sepsis. January 2017, http://apps.who.int/gb/ebwha/pdf_files/EB140/B140_R5-en.pdf.
- Keel M, Harter L, Reding T, et al. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit Care Med. 2009;37(5):1642-1648.
- Llewelyn MJ, Berger M, Gregory M, et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit. Care. 2013;17:R60, doi: 10.1186/cc12588.
- Que YA, Delodder F, Guessous I, et al. Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management. Crit Care 2012;16:R114.
Que YA, Guessous I, Dupuis-Lozeron E, et al. Prognostication of mortality in critically ill patients with severe infections. Chest. 2015;148(3):674-682. - García de Guadiana-Romualdo L, Berger M, Jiménez-Santos E, et al. Pancreatic stone protein and soluble CD25 for infection and sepsis in an emergency department, Eur J Clin Invest. 2017;47(4):297–304.
- Klein HJ, Csordas A, Falk V, et al. Pancreatic stone protein predicts postoperative infection in cardiac surgeryp atients irrespective of cardiopulmonary bypass or surgical technique. PLoS ONE. 2015;10(3):e0120276.
- Xin Z, Dan L, You-Ning L, Rui W, Li-Xin X. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: a meta-analysis. Crit Care. 2015;19:323.
- Dan L, Lixin X, Haiyan Z, Xueyao L, Jie C, Prognostic value of mid-regional proadrenomedullin (MR-proADM) in patients with community-acquired pneumonia: a systematic review and meta-analysis. BMC Infectious Diseases 2016;16:232.
- Linder A, Arnold R, Boyd JH et al. Heparin-binding protein measurement improves the prediction of severe infection with organ dysfunction in the Emergency Department, Crit Care Med. 2015;43(11):2378-2386.
- Durand N, Saveriades E, Renaud P. Detecting proteins complex formation using steady-state diffusion in a nanochannel. Anal Bioanal Chem. 2009;394(2):421–425.
Fabien Rebeaud, PhD, serves as Chief Scientific Officer of Lausanne, Switzerland-based Abionic, which has developed and commercialized abioSCOPE, a rapid point-of-care diagnostic platform to improve medical diagnosis. A test for sepsis measuring PSP is scheduled for launch in hospital emergency departments and intensive care units in 2018.