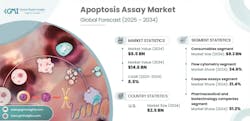
In 2024, the global apoptosis assay market stood at USD 6.5 billion and is forecast to grow from USD 7 billion in 2025 to USD 14.6 billion by 2034, representing a compound annual growth rate of 8.5 percent.
This upward trend is being supported by a rising incidence of chronic diseases, increasing use of personalized medicine, and ongoing improvements in assay technology. A major contributor to market growth is the increasing occurrence of chronic illnesses such as cancer, neurological disorders, and autoimmune diseases. Apoptosis assays are essential for studying the role of abnormal cell death in these conditions and for determining whether treatments can selectively destroy harmful cells while sparing healthy ones.
The rising emphasis on personalized medicine is another powerful driver. These assays allow for the analysis of patient-specific cellular reactions, making it possible to match therapies to individual needs and improve outcomes. This shift toward tailored healthcare is steadily increasing demand for precise cell-based testing methods.
Technological progress in flow cytometry and imaging is enhancing the capabilities of apoptosis detection. Modern systems now deliver higher speed, greater accuracy, and improved scalability, enabling large-scale testing without sacrificing reliability. Laboratories can now process more samples and obtain richer data sets in less time.
The market is also benefiting from broader use in toxicology and safety testing. Detecting drug-induced cell death in early development stages can help pharmaceutical companies reduce safety risks, avoid late-stage trial failures, and comply with regulatory expectations. This application is especially valuable in preclinical screening, where early warning signs can guide critical go/no-go decisions.
The high cost of advanced apoptosis assay technologies remains a hurdle, particularly for smaller biotech firms and cost-conscious research facilities. These expenses can limit adoption in regions with constrained healthcare budgets.
Regulatory complexity and ethical considerations also pose challenges. Approval processes for studies involving cell death pathways can be time-consuming, and varying international regulations may slow product launches or research collaborations.
Despite these obstacles, the market outlook remains positive. Innovation is expected to bring down costs and make apoptosis assays more accessible to a wider range of users. As pharmaceutical companies, biotechnology firms, and research institutions continue to invest in advanced tools for disease research and safety assessment, adoption will broaden.
The combination of an aging global population, increasing prevalence of chronic diseases, and growing reliance on personalized healthcare ensures that apoptosis assays will remain a critical part of biomedical research. Over the next decade, these assays are likely to become even more embedded in therapeutic development and safety evaluation, reinforcing their role in advancing precision medicine.