The placenta is an ephemeral organ that provides us with the nutrients necessary for development and our initial immunity, removes malevolent agents, and much more before being cast off as we enter the world. Though all organs work together to support life, as our first organ, the significance of the placenta is irrefutable. Placental dysfunction, along with several other factors, is known to contribute to the development of pre-eclampsia — a complex, multisystem hypertensive disorder of pregnancy. While the etiology of pre-eclampsia remains largely unknown, the grave complications associated with it have driven development of novel methods for predicting its onset.
The placenta is an ephemeral organ that provides us with the nutrients necessary for development and our initial immunity, removes malevolent agents, and much more before being cast off as we enter the world. Though all organs work together to support life, as our first organ, the significance of the placenta is irrefutable. Placental dysfunction, along with several other factors, is known to contribute to the development of pre-eclampsia — a complex, multisystem hypertensive disorder of pregnancy. While the etiology of pre-eclampsia remains largely unknown, the grave complications associated with it have driven development of novel methods for predicting its onset.
Pre-eclampsia and epidemiology
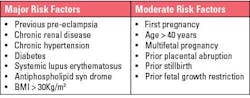
Pre-eclampsia is traditionally defined as new onset hypertension and proteinuria in pregnancy,1 however, the International Federation of Gynecology and Obstetrics’ (FIGO) clinical definition describes it as sudden onset hypertension (>20 weeks of gestation) and at least one of the following: proteinuria, maternal organ dysfunction, or uteroplacental dysfunction.2 It is responsible for an estimated 70,000 maternal deaths, and 500,000 fetal deaths globally.3 Pre-eclampsia affects around 4% of pregnancies in the United States and is more common in low-to-middle income countries (LMICs), displaying an overall pooled incidence of 13% in a cohort from sub-Saharan Africa.4 The risk factors for pre-eclampsia are shown in Figure 1.
Pre-eclampsia is associated with increased morbidity and mortality worldwide. In the United States, pre-eclampsia is the foremost cause of maternal death, severe maternal morbidity, maternal intensive care admissions, and premature births.5
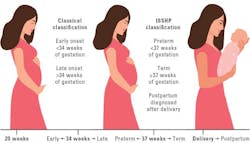
Classical classification of pre-eclampsia included early-onset (<34 weeks gestation) and late-onset (>34 weeks gestation). However, this classification lacks clinical utility as they do not accurately illustrate maternal or fetal prognosis. Therefore, the International Society for the study of Hypertension in Pregnancy (ISSHP) and contemporary studies prefer to classify pre-eclampsia as preterm (delivery <37 weeks of gestation), term (delivery ≥37 weeks of gestation) and postpartum pre-eclampsia (after delivery) (Figure 2).
Complications
Pre-eclampsia has been associated with acute and chronic complications for both mother and child. Worldwide risk of maternal and fetal morbidity displays adjusted odds ratios of 3.73 and 3.12 respectively (pre-eclampsia versus non pre-eclampsia).6
Acute maternal complications
A range of neurological complications are associated with pre-eclampsia. The most obvious is eclampsia, defined as seizures in pregnant women commonly from 20 weeks of gestation or after birth.7 Eclampsia has two proposed mechanisms: abnormal placentation reduces blood supply and causes oxidative stress, leading to endothelial damage; and elevated blood pressure in pre-eclampsia disrupts cerebral vasculature, causing hypoperfusion and damage.8 In high-income countries (HICs), most women make a full recovery, however, more severe cases of eclampsia can result in permanent disability or brain damage.7
Stroke is a significant complication of pre-eclampsia, constituting 36% of strokes related to pregnancy.9 The hypertension characteristic of pre-eclampsia can weaken the walls of blood vessels causing subarachnoid or intracerebral haemorrhage resulting in haemorrhagic stroke. Ischaemic stroke is also of concern due to blood clotting complications, which will be discussed later.
Additional neurological complications include visual scotoma, cortical blindness, cerebral venous sinus thrombosis, cerebral vasoconstriction syndrome, and posterior reversible encephalopathic syndrome (PRES). Notably, the last three in this list frequently manifest postpartum without warning.6
HELLP (haemolysis, elevated liver enzymes, and low platelets) syndrome is a liver and blood clotting disorder and life-threatening complication of pre-eclampsia. HELLP syndrome most commonly presents immediately postpartum but can manifest any time after 20 weeks of gestation.7 Microangiopathy, or small blood vessel disorder, leads to ischaemia and a subsequent increase in oxidative stress and inflammation, causing an increase in liver enzymes and participates in the initiation of HELLP. Thrombocytopenia, or platelet deficiency, is considered a product of platelet depletion resulting from heightened platelet activation triggered by widespread endothelial damage.6
Another blood clotting condition associated with pre-eclampsia is disseminated intravascular coagulation (DIC),7 described as the dysfunction of the maternal blood clotting system resulting in multiple organ dysfunction syndrome.10 DIC can cause excessive bleeding due to lack of clotting proteins, or the formation of clots due to overactive clotting proteins, ultimately causing organ damage.10
As described earlier, proteinuria is included in the diagnostic criteria for pre-eclampsia, suggesting involvement of the kidneys. This is caused by high concentrations of soluble FMS like tyrosine kinase 1 (sFLT-1), a placental angiogenic factor, which inhibits proteins of the podocyte slit diaphragm, which is involved in preventing the leakage of proteins into the urine.6,11 Reduced levels of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) stimulates endothelin-1 expression,6 known to promote podocyte detachment, further contributing to proteinuria.12
Finally, pulmonary edema, excessive fluid accumulation in the lungs, is an acute and life-threatening complication associated with pre-eclampsia, the likelihood of which is increased via administration of antihypertensive medications.6
Acute neonatal complications
There are several documented complications affecting the baby of a pre-eclamptic mother. Firstly, intrauterine growth restriction (IUGR) can result in underdevelopment of the fetus because of deficient transfer of oxygen and other nutrients from mother to child.13 This can result in low birth weight, particularly when pre-eclampsia occurs prior to 37 weeks of gestation.7 In pre-eclampsia with severe symptoms, delivery frequently occurs prematurely, either spontaneously or through induction. Preterm delivery can result in complications such as neonatal respiratory distress syndrome and neonates requiring ICU admission.7 Additionally, there is increased risk of stillbirth in pre-eclamptic pregnancies with relative risk shown to be 1.45 (95% Cl 1.20-1.76).14 Other complications documented in neonates born through pre-eclamptic pregnancies include neonatal thrombocytopenia, bronchopulmonary dysplasia, and a range of neurodevelopment outcomes.15
Long-term complications
The only known cure for pre-eclampsia is delivery. However, the complications for both mother and child can last long after even an uncomplicated delivery. After a pre-eclamptic pregnancy, women are at increased risk of end stage renal disease (4.7-fold), stroke (4-fold), and vascular dementia (3-fold) later in life.5 Women are also at increased risk of other cardiovascular diseases (CVD) including chronic hypertension, coronary artery disease, congestive heart failure, and ischaemic heart disease.5,13 In offspring, IUGR increases the risk of development of hypertension and other CVDs.13 Finally, offspring have been shown to be at higher risk of increased body mass index, changes in neuroanatomy, reductions in cognitive function, and hormonal abnormalities.13
sFLT-1/PlGF ratio
The pathophysiology of pre-eclampsia is complex and enigmatic. However, placental dysfunction is known to be a factor in pre-eclampsia development. The placental-related angiogenic factors, sFLT-1 (anti-angiogenic), and PlGF (pro-angiogenic) have been implicated in this development. This ratio provides a useful measure of placental dysfunction as a sharp increase in sFLT-1 and decrease in PlGF has been shown approximately 5 weeks before onset of pre-eclampsia.16
Until recently, diagnosis of pre-eclampsia was one of clinical manifestation. However, studies such as PROGNOSIS17 and PROGNOSIS Asia,18 along with others,19,20 have shown strong utility of this ratio. The PROGNOSIS study showed that a ratio cutoff of ≥38 was useful for ruling out pre-eclampsia within one week with a negative predictive value (NPV) of 99.3% or four weeks with a positive predictive value (PPV) of 36.7%.17 The definitions of pre-eclampsia used by the International Society for the Study of Hypertension in Pregnancy (ICCHP) and the American College of Obstetricians and Gynaecologists (ACOG) have a PPV of around 20%, but when used in combination with the sFLT-1/PlGF ratio, the PPV is enhanced to 65.5% for ruling in pre-eclampsia within four weeks.21
Similar results have been shown in an Asian cohort in the PROGNOSIS Asia Study. Using the same cutoff value, this study reported an NPV of 98.9%.18 Furthermore, in a sub-analysis of this cohort that looked at Japanese participants, a cutoff of ≥38 displayed an NPV of 100% for ruling out pre-eclampsia within one week and a PPV of 32.4% for ruling in within four weeks.22
Accurate identification is essential
Like all clinical assays, those used to determine the sFLT-1/PlGF ratio are subject to rigorous quality control, essential to ensure accurate results and diagnosis. The complications of pre-eclampsia are severe and often life-threating for both mother and child. Early and accurate identification is imperative for optimal monitoring, management, and timely interventions to reduce the risk of the grave consequences associated with pre-eclampsia.
The utility of the sFLT-1/PlGF ratio has been shown over various large cohorts and provides improved identification when used in combination with established clinical definitions. While the enigma of pre-eclampsia persists, the dedication of the scientific community to unravel its complexities ensures a future where expectant mothers may benefit from more effective and tailored strategies to mitigate the risks associated with this puzzling condition. Continued research endeavours will undoubtedly shape the landscape of maternal-fetal medicine, fostering advancements that hold the promise of improved outcomes for both mothers and their unborn children.
References
1. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131. doi:10.1097/01.AOG.0000437382.03963.88.
2. Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre‐eclampsia: A pragmatic guide for first‐trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(S1):1-33. doi:10.1002/ijgo.12802.
3. Karrar SA, Hong PL. Preeclampsia. StatPearls Publishing; 2023.
4. Jikamo B, Adefris M, Azale T, Alemu K. Incidence, trends and risk factors of preeclampsia in sub-Saharan Africa: a systematic review and meta-analysis. PAMJ - One Health. 2023;11:1. doi:10.11604/pamj-oh.2023.11.1.39297.
5. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia. Circ Res. 2019;124(7):1094-1112. doi:10.1161/CIRCRESAHA.118.313276.
6. Dimitriadis E, Rolnik DL, Zhou W, et al. Pre-eclampsia. Nat Rev Dis Primers. 2023;9(1):8. doi:10.1038/s41572-023-00417-6.
7. NHS. Pre-eclampsia. Health A to Z. Published September 28, 2021. Accessed January 3, 2024. https://www.nhs.uk/conditions/pre-eclampsia/complications/.
8. Magley M, Hinson MR. Eclampsia. StatPearls Publishing; 2023.
9. Crovetto F, Somigliana E, Peguero A, Figueras F. Stroke during pregnancy and pre-eclampsia. Curr Opin Obstet Gynecol. 2013;25(6):425-432. doi:10.1097/GCO.0000000000000024.
10. Costello RA, Nehring SM. Disseminated Intravascular Coagulation. StatPearls Publishing; 2023.
11. Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol. 2020;24(3):193-204. doi:10.1007/s10157-020-01854-3.
12. Trimarchi H. Mechanisms of Podocyte Detachment, Podocyturia, and Risk of Progression of Glomerulopathies. Kidney Dis (Basel). 2020;6(5):324-329. doi:10.1159/000507997.
13. Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am J Physiol Renal Physiol. 2020;1;318(6):F1315-F1326. doi:10.1152/ajprenal.00071.2020.
14. Harmon QE, Huang L, Umbach DM, et al. Risk of fetal death with preeclampsia. Obstet Gynecol. 2015;125(3):628-635. doi:10.1097/AOG.0000000000000696.
15. Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal Preeclampsia and Neonatal Outcomes. J Pregnancy. 2011;2011:1-7. doi:10.1155/2011/214365.
16. Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202(2):161.e1-161.e11. doi:10.1016/j.ajog.2009.09.016.
17. Zeisler H, Llurba E, Chantraine F, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med. 2016;7;374(1):13-22. doi:10.1056/NEJMoa1414838.
18. Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74(1):164-172. doi:10.1161/HYPERTENSIONAHA.119.12760.
19. Hughes RCE, Phillips I, Florkowski CM, Gullam J. The predictive value of the sFlt‐1/PlGF ratio in suspected preeclampsia in a New Zealand population: A prospective cohort study. Aust N Z J Obstet Gynaecol. 2023;63(1):34-41. doi:10.1111/ajo.13549.
20. Nikuei P, Rajaei M, Roozbeh N, et al. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth. 2020;20(1):80. doi:10.1186/s12884-020-2744-2.
21. Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022;27:42-50. doi:10.1016/j.preghy.2021.12.003.
22. Ohkuchi A, Saito S, Yamamoto T, et al. Short-term prediction of preeclampsia using the sFlt-1/PlGF ratio: a subanalysis of pregnant Japanese women from the PROGNOSIS Asia study. Hypertens Res. 2021;44(7):813-821. doi:10.1038/s41440-021-00629-x.
About the Author

Jason Armstrong
BSc is a Scientific Content Creator at Randox Laboratories Ltd. After achieving his degree in Biochemistry and beginning his career in an R&D laboratory, he made the switch to pursue his passion in scientific communications.