Cross-reactive carbohydrate determinants — history and relevance in allergy diagnostics
The story of cross-reactive carbohydrate determinants (CCDs) is a fascinating journey that spans over 30 years of research and takes us on a global adventure. Historically, CCDs are a confounding factor that can lead to false-positive blood test results in up to 30% of patients allergic to plants or insect venoms.1 However, recent research has shed new light on the relevance of CCDs in the field of carbohydrate epitopes in allergy: remnant CCDs on solid-phase in vitro diagnostic technologies and their impact on accurate allergy diagnosis.
CCDs – a brief history
Let’s start at the beginning, about 30 years ago, when a group of scientists in the Netherlands made a remarkable discovery in the study of allergies. They observed that some patient sera reacted to an array of allergen extracts, including pollen, food, and insect venoms. To investigate this phenomenon, the scientists incubated the extracts with periodate, a reagent used to break down terminal sugar residues in carbohydrates. Their findings revealed a significant reduction in reactivity, thus establishing, for the first time, a link between carbohydrate epitopes and allergy diagnostics.2
Since then, much has been discovered about CCDs, including the fact that a partial motif of certain glycosylations of some plant and insect allergens, a core α-1,3-fucose, is responsible for the observed cross-reactivity3,4. However, the clinical relevance of sensitization to CCDs has been a longstanding issue that still persists to some extent. Several studies, mostly based on functional assays such as basophil activation tests (BAT), have been performed to obtain an unambiguous answer.5,6,7,8,9 After many years of careful observation, it is now generally accepted that immunoglobulin E (IgE) directed against CCDs cannot cause allergic symptoms. Yet, anti-CCD IgE plays a relevant role in allergy diagnostics.
CCDs in modern allergy in vitro diagnosticsBlood tests in allergy diagnostics measure the presence of IgE directed against allergens. If a clinically non-relevant, cross-reactive IgE epitope is present on the surface of an allergen, a false-positive result can complicate the diagnosis in patients with matching IgE. In the worst case, incorrect diagnoses are made, and the wrong therapy is initiated.
To address this problem, physicians have several options, including in vivo allergy testing (with the risk of inducing a severe systemic reaction in patients), the use of cellular assays (such as the labor-intensive BAT), component-resolved diagnostics (CRD), or the inhibition of anti-CCD IgE with artificial CCD inhibitors. However, each of these options comes with its specific set of caveats, such as the increased risk of systemic reactions, sampling issues, or cost-efficiency.
Over the past two decades, the use of molecular allergen components (i.e., CRD) has become more established as a means of choice in allergy diagnostics. Recombinant, CCD-free components that are not affected by the anti-CCD IgE in patient sera can be produced using artificial expression systems. CRD not only solved the CCD issue but also enabled physicians to assess the risk and severity of a potential allergic response.
All’s well that ends well? Well, according to a 2009 study conducted by French physicians and scientists, the answer is no. The study compared solid-phase and liquid-phase allergy diagnostic systems in patients with peanut and pollen allergies and found that the solid-phase system produced twice as many false-positive results as the liquid-phase technology. This was also reflected in the significantly higher specificity of the liquid-phase system. The authors were also able to link the false-positive results on the solid-phase platform to CCD sensitization in individual patients.10
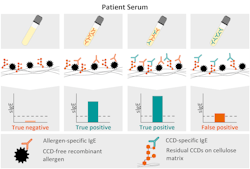
However, it was not until 2018 that Austrian scientists identified the cause of diverging results in solid- and liquid-phase technologies. The solid-phase system, most frequently used in allergy diagnostics, is based on a cellulose matrix that can have remnant proteins with CCDs on its surface, introducing this confounding factor into the underlying assay design. The authors tested different allergy patient groups on a solid-phase system that was utilizing a cellulose matrix before and after incubating patient sera with CCD inhibitors. This study confirmed that CCD sensitized patients are more prone to be diagnosed with artificially elevated IgE titers or even false-positive results on solid-phase systems even if tested with an “empty” (i.e., allergen free) test or recombinant, CCD-free components (Figure 1).11With more than 100 million people affected by allergies in the United States alone,12,13 a reliable diagnostic tool is crucial. Statistically speaking, 25 million allergic patients in the United States are sensitized to CCDs and therefore at risk of being impacted by the diagnostic pitfalls of cellulose-based, solid-phase technologies.
The million-dollar question is, do liquid-phase systems provide a viable alternative? Researchers from the United States tackled this challenge head-on in 2020 with the assistance of Johns Hopkins University. The study compared the impact of CCD inhibition on IgE results generated on solid-phase and two liquid-phase platforms. The results showed that liquid-phase technologies are less susceptible to CCD interference, with minimal reductions in IgE titers post-CCD inhibition. In contrast, the solid-phase system experienced a significant impact, providing additional evidence of remnant CCDs on the cellulose matrix.14 The liquid-phase systems utilized in this study are based on CCD-free microparticles and beads, making them less susceptible to anti-CCD IgE.
Conclusion
In conclusion, the story of CCDs is a tale of scientific discovery and technological advancement that spans over three decades. While CCDs were initially identified as a confounding factor in allergy diagnostics, recent research has revealed the importance of re-addressing this issue to ensure accurate diagnoses and appropriate treatments for patients. The development of CRD has revolutionized allergy diagnostics, allowing for the production of CCD-free allergen components that do not interfere with anti-CCD IgE in patient sera. However, the solid-phase IVD technologies often used in allergy diagnostics have introduced new challenges, with remnant CCDs on cellulose matrices leading to elevated or even false-positive results in patients sensitized to CCDs. The evidence suggests that liquid-phase technologies offer a viable alternative, with CCD-free microparticles and beads proving to be less susceptible to anti-CCD IgE interference.
With millions of people affected by allergies worldwide, the accurate diagnosis of allergic sensitization is essential to ensure the appropriate management of symptoms and to prevent potentially life-threatening reactions. The ongoing research into CCDs and their impact on allergy diagnostics highlights the importance of continuing scientific inquiry and technological innovation to improve patient outcomes in allergy management.
REFERENCES
1. Holzweber F, Svehla E, Fellner W, et al. Inhibition of IgE binding to cross‐reactive carbohydrate determinants enhances diagnostic selectivity. Allergy. 2013;68(10):1269-77. doi:10.1111/all.12229.
2. Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68(5):356-64. doi:10.1016/0091-6749(81)90133-0.
3. Kurosaka A, Yano A, Itoh N, Kuroda Y, Nakagawa T, Kawasaki T. The structure of a neural specific carbohydrate epitope of horseradish peroxidase recognized by anti-horseradish peroxidase antiserum. J Biol Chem. 1991;5;266(7):4168-72.
4. Bencúrová M, Hemmer W, Focke-Tejkl M, Wilson IB, Altmann F. Specificity of IgG and IgE antibodies against plant and insect glycoprotein glycans determined with artificial glycoforms of human transferrin. Glycobiology. 2004;14(5):457-66. doi:10.1093/glycob/cwh058.
5. van der Veen MJ, van Ree R, Aalberse RC, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100(3):327-34. doi:10.1016/s0091-6749(97)70245-8.
6. Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129(4):286-95. doi:10.1159/000067591.
7. Fötisch K, Altmann F, Haustein D, Vieths S. Involvement of carbohydrate epitopes in the IgE response of celery-allergic patients. Int Arch Allergy Immunol. 1999;120(1):30-42. doi:10.1159/000024217.
8. Foetisch K, Westphal S, Lauer I, et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003;111(4):889-96. doi:10.1067/mai.2003.173.
9. Wicklein D, Lindner B, Moll H, et al. Carbohydrate moieties can induce mediator release: a detailed characterization of two major timothy grass pollen allergens. Biol Chem. 2004;385(5):397-407. doi:10.1515/BC.2004.044.
10. Guilloux L, Morisset M, Codreanu F, Parisot L, Moneret-Vautrin DA. Peanut allergy diagnosis in the context of grass pollen sensitization for 125 patients: Roles of peanut and cross-reactive carbohydrate determinants specific IgE. Int Arch Allergy Immunol. 2009;149(2):91-7. doi:10.1159/000189190.
11. Ng A, Boersma P. Diagnosed Allergic Conditions in Adults: United States, 2021. National Center for Health Statistics (U.S.); 2023.
12. Zablotsky B, Black L, Akinbami L. Diagnosed Allergic Conditions in Children Aged 0–17 Years: United States, 2021. National Center for Health Statistics (U.S.); 2023.
13. Hemmer W, Altmann F, Holzweber F, Gruber C, Wantke F, Wöhrl S. ImmunoCAP cellulose displays cross-reactive carbohydrate determinant (CCD) epitopes and can cause false-positive test results in patients with high anti-CCD IgE antibody levels. J Allergy Clin Immunol. 2018;141(1):372-381.e3. doi:10.1016/j.jaci.2017.04.028.
14. Sinson E, Ocampo C, Liao C, et al. Cross-reactive carbohydrate determinant interference in cellulose-based IgE allergy tests utilizing recombinant allergen components. PLoS One. 2020;23;15(4):e0231344. doi:10.1371/journal.pone.0231344.
About the Author

Johannes Grosch, Dr. rer. nat.
is one of the specialists for allergy laboratory diagnostics at Siemens Healthineers. He holds a B.Sc. in Nutritional Sciences from the University of Vienna, an M.Sc. in Biochemistry from the Technical University of Braunschweig, and finished his doctorate in Molecular Allergology (Technical University of Munich) in 2022. In his role, Johannes is responsible for educating and collaborating with healthcare professionals, allergists, and laboratorians.

Friedrich Altmann, Dr. nat. techn.
is a biochemist with an extensive track record in the field of glycobiology and glycoimmunology. With more than 400 peer-reviewed publications, he is one of the leading experts on clinically relevant glycosylations. He holds a full professorship at the University of Natural Resources and Life Sciences, Vienna.