The right test at the right time during the SARS-CoV-2 pandemic
The SARS-CoV-2 pandemic has had a grip on the world since the virus first appeared in December of 2019. Months later, professionals across the scientific and medical community have had to endure challenges that they may not have faced in their careers before now. With these new challenges come new ways of thinking and approaching different facets of the clinical cascade when caring for patients with COVID-19. Many countries have had to adapt their clinical practices to ever-changing guidelines, therapies, and approaches to the standard of care in order to help patients with a range of ailments.
As the pandemic has forged through 2020, the knowledge gap has begun to close, helping the medical and scientific community better care for patients with different levels of infection. This gap may be widened again when the pandemic collides with the upcoming respiratory season, which presents many unknowns.
In March of 2020, when much of the United States went into lockdown due to the pandemic, one thing became clear: the incidence of influenza cases declined sharply in just a few weeks.1 However, during a nationwide lockdown where many additional precautions were in effect (i.e. mask-wearing, physical and social distancing) this could have been expected. With the 2020/2021 respiratory illness season on the horizon, while the world continues to experience increasing SARS-CoV-2 case counts, the United States, along with many other countries, is preparing for what might be an arduous season of respiratory illness in the face of pandemic fatigue.
Given that clinical laboratories have grappled with testing for SARS-CoV-2 throughout the pandemic, questions arise about what to do when other pathogens, especially influenza, begin to circulate again during the winter months. Labs have had to focus on testing for SARS-CoV-2 for months, bringing on multiple testing platforms and adding new tests onto existing platforms to keep up with testing demand. What this will mean as far as testing needs for influenza and other respiratory pathogens (e.g. rhinovirus) is still a blurry picture. Clinical laboratories and medical teams face even further challenges in the wake of economic reopening, which includes children and young adults returning to schools and colleges.
During a normal season of respiratory illness, influenza, or the flu, is a common household term. The incidence generally remains high year-over-year, despite the availability of antivirals and an influenza vaccine. Previously, any set of signs and symptoms may have been referred to as the flu, or maybe even just a cold if the symptoms were not as severe, but now the ramifications of not knowing the underlying cause of the infection could be much greater. The likelihood that an illness is either the flu or a cold is certainly plausible, but clinians should consider identifying the other pathogens, especially when a SARS-CoV-2 or influenza test result turns up negative.
The importance of testing for other respiratory pathogens
Other respiratory pathogens, which may have been dormant from the tail end of the winter through the summer, now have the opportunity to start circulating again. All the while, SARS-CoV-2 has not appeared to be seasonal – at least thus far.
When it comes to respiratory infections, it may be more important than ever during this season to understand when the etiology of infection is SARS-CoV-2 but also whether other important respiratory agents are playing a role in transmission and infection. The clinical presentation of respiratory infections and most of the symptoms a person may experience are widely similar, complicating diagnosis in the absence of a SARS-CoV-2-positive result, especially for those patients with underlying comorbidities.
Outside of influenza and SARS-CoV-2 lies a full spectrum of viruses and bacteria that cause acute upper respiratory tract infections. For example, another widely known virus, respiratory syncytial virus (RSV), has been associated with mortality rates up to 60 percent in untreated immunocompromised individuals. Although more commonly seen in the pediatric population, RSV has also caused severe outcomes in immunocompromised adults, especially the elderly and certain comorbidities could lead to a significant increase in the risk of hospitalization.2 Furthermore, identifying the subtype of RSV as A or B could be of significance. Laham et al., for example, described a handful of studies where RSV A had propagated worse clinical outcomes in pediatric patients. In their study, RSV A had a higher severity of illness when certain confounding factors, like co-infection with another respiratory pathogen, were removed.3
Parainfluenza (PIV) has also been known to affect multiple age groups, with the more distressing cases of disease severity in younger children, older adults, and immunocompromised individuals. Lung and stem cell transplant recipients are a particularly high-risk population for infection with PIV. Each serotype has the potential to produce specific clinical symptoms and can be found circulating at different times throughout the year, making them a cause for concern both in and out of the common high-circulation months for most respiratory pathogens. Recent findings have shown that in immunocompromised adults, hospitalized adult pneumonia cases caused by PIV were associated with comorbidities, like chronic lung or heart disease. The importance of identifying this pathogen and its different serotypes could play a role for the clinical team in managing adult or pediatric patients, as PIV-3 has been seen to be more prevalent over PIV-1 and PIV-2 and has been associated with critical diseases including pneumonia and bronchiolitis.4
Since the 2003 coronavirus outbreak, which caused severe acute respiratory syndrome (SARS), the four common human coronaviruses (229E, HKU1, NL63, and OC43) have not received much publicity. However, these coronaviruses hold the ticket to 15-30 percent of respiratory tract infections each year, causing more severe infections in neonates, the elderly, and those with underlying conditions.5 The other two well-known coronaviruses, SARS-CoV and Middle East Respiratory Syndrome Coronavirus (MERS-CoV), are recognized for their above average mortality rates, although only MERS-CoV is still circulating.
Rhinovirus is another contender. With over 100 serotypes, it may account for up to one-third of “common cold” cases. While healthy individuals generally may not suffer a great disease burden from rhinoviruses, other individuals like those in long term care facilities may be at an increased risk for poor outcomes. Louie et al. described a rhinovirus outbreak between two long term care facilities in California, resulting in an attack rate of 100 percent and a mortality rate of 21 percent, with all the deaths being directly attributed to the acute infection.6 Human metapneumovirus (hMPV) wreaked similar havoc in skilled nursing facilities in 2013, where the viral etiology was originally thought to be influenza. There was a total of 57 cases of upper respiratory tract infection, which were all caused by hMPV, and 11 percent of the patients at the two facilities died.7
Adenoviruses can be of particular concern for group settings as well. A recent outbreak that made news headlines occurred at the Wanaque Center for Nursing and Rehabilitation. During the time of the outbreak, a total of 36 children and 1 staff member were infected with adenovirus type 7, which has been linked to poor outcomes in patients with weakened immune systems. The adenovirus spread resulted in 11 untimely pediatric deaths.8 Likewise, enteroviruses (EV) have been linked to poor outcomes in children. The viruses can cause not only upper respiratory tract infection but also lead to acute flaccid myelitis (AFM), which can rapidly progress to poor outcomes including paralysis and/or complications of respiratory failure that can be life-threatening even in healthy children. The most recent guidance from the Centers for Disease Control and Prevention (CDC) said that 2020 may be another peak year for AFM, as EV-D68 has circulated every two years between August and November since 2014 and is likely associated with a peak in AFM cases during these periods. The 2018 season highlights the importance of diagnostic testing for EV, as most children with limb weakness had a fever and/or respiratory illness before onset.9
While many more viruses can cause upper respiratory illness, viruses are not the only offenders causing varying levels of respiratory distress. Bacteria, such as Chlamydia pneumoniae and Mycoplasma pneumoniae, also play a role in atypical, community-acquired pneumonia with symptoms consistent with infections caused by some of the viruses previously described. The difference with these pathogens is that an antibiotic prescription is recommended for treatment in any population. Individuals with asthma or atopic disease that may have a less than optimal ability to produce an immune response, for example, have a substantial foe in M. pneumoniae, which has evolved to induce long-lasting infection.10
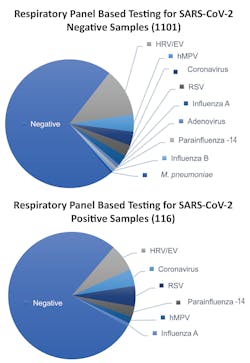
The clinical implications for co-infections do not appear to be widely studied and leave a bit of a knowledge gap, but we do know based on some studies that co-infections are a possibility with and without SARS-CoV-2 infection. Researchers from Stanford University School of Medicine recently published a research letter in JAMA detailing the prevalence of SARS-CoV-2 and other circulating respiratory pathogens, based on results from SARS-CoV-2 testing and a syndromic multiplex respiratory panel with targets spanning many different viruses and bacteria (See Figure 1). The authors found that infection with SARS-CoV-2 could not predict the presence or absence of another respiratory pathogen, which could have important implications for the upcoming respiratory season.11 Another recently published study by Mandelia et. al., noted that viral co-infection epidemiology has been poorly understood, in general, even before the pandemic. However, of the samples that tested positive in their study, 10.8 percent were positive for more than one virus, with the pediatric patient population carrying a larger burden of co-infections and adenovirus being the most prevalent co-detected pathogen.12
The etiology of a respiratory tract infection can be challenging to differentiate clinically. Infections with one or more pathogens generally cannot be diagnosed by symptoms only, further complicating patient management and treatment. Being able to identify the causative agent(s) in an upper respiratory tract infection can be useful information in the clinical management of any patient. With the inability to predict how effective the influenza vaccine will be over a specific flu season, determining whether a patient has been infected with influenza will be key to properly prescribing an antiviral in the appropriate timeframe. Likewise, if a bacterial pathogen is detected, an antibiotic might need to be administered to help curb the infection. Given that there are no other widely disseminated treatments for other viral pathogens, prescription of an antiviral or antibiotic likely will be given preemptively or under clinical suspicion when it may not be needed.
Combating antimicrobial resistance
While much of the public has been focused on the pandemic, the scientific and medical community has not forgotten about the major burden of antimicrobial resistance (AMR) worldwide. A recent Clinical and Laboratory Standards Institute News Update explored what we know about AMR during the COVID-19 pandemic, including the widespread use of antibiotics for presumed or confirmed secondary bacterial infections, and what the clinical microbiology lab can do to help prepare for the AMR pandemic with key activities.13
One of the suggestions that the authors in the update made is for the rapid detection of respiratory pathogens, which can be done using syndromic multiplex molecular respiratory panels.13 The CDC also commented that infection with SARS-CoV-2 and other pathogens has been reported and that detection of one pathogen does not indicate there is not an infection with SARS-CoV-2 as well.,14 Rapid identification of these pathogens can help providers administer proper antiviral and/or antibacterial therapy in an actionable timeframe, or avoid giving an antibiotic or antiviral altogether, which can aid in the fight against AMR. Even if therapy has been started, some studies have said that stopping unnecessary therapy early may be linked to shrinking drug resistance.15 These panels can also play a critical role in infection control and hospital admissions.
Larger diagnostic panels can aid in determining the cause of respiratory infection in many ways. For instance, a rapid result for a normally healthy person may allow for decreased hospital admissions, as seen in a recent publication where 8.4 percent of adults were not admitted to the hospital because of the rapid diagnostic test result, which ultimately saved the hospital time and made beds available for sicker patients.16 Earlier results from a rapid multiplex panel can allow hospitals to focus on patients that need to be admitted and potentially segregated to a designated COVID-19 ward/area or triaged to another area due to other known respiratory illnesses. This will be crucial to help ensure that patients – especially those undergoing surgeries, in recovery, and with underlying comorbidities – are not exposed to SARS-CoV-2 or any of the other respiratory pathogens discussed here. Likewise, hospital patients being discharged to rehabilitation or long term care facilities who have a negative SARS-CoV-2 test result and have respiratory symptoms may need to be assessed other respiratory illnesses.
Matching the test to the care setting
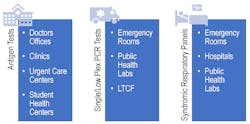
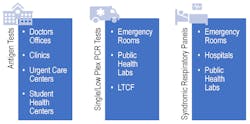
Now that options for SARS-CoV-2 and influenza diagnosis are more accessible through avenues such as antigen testing, it will be important to determine which type of test is needed for different patient groups (See Figure 2). It remains to be seen how antigen-based tests will play a role during the upcoming respiratory season and where they best fit into the clinical care pathway.
In the past, antigen testing for influenza has not been deemed the gold standard due to the low sensitivity and specificity for these tests, driving conversion in many laboratories to molecular-based methods.17 Additionally, these viral antigen-based tests cannot rule out infection with other agents, an important consideration for certain populations, such as those with any comorbidities. During the upcoming respiratory season, as the scientific and medical community prepares to line the battlefield for an uncertain future, there is a definite need to ensure they are armed with the right test, at the right time, to provide the clearest clinical picture, especially for patients at a higher risk of clinical complications.
References:
- Influenza Positive Tests Reported to CDC by Clinical Laboratories, National Summary,2019-20 Season, week ending Sep 12, 2020. Centers for Disease Control and Prevention. September 2020. https://www.cdc.gov/flu/weekly/index.htm. Accessed September 30, 2020.
- Chatzis O, Darbre S, Pasquier J, et al. Burden of severe RSV disease among immunocompromised children and adults: a 10-year retrospective study. BMC Infect Dis. 2018 Mar 6;18(1):111. doi: 10.1186/s12879-018-3002-3.
- Laham FR, Mansbach JM, Piedra PA, et al. Clinical profiles of respiratory syncytial virus subtypes A and B among children hospitalized with bronchiolitis. Pediatr Infect Dis J. 2017;36(8):808-810. doi:10.1097/INF.0000000000001596.
- Howard LM, Edwards KM, Yuwei Zhu Y, et al. Parainfluenza virus types 1-3 iInfections among children and adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2020 Jul 18; doi.org/10.1093/cid/ciaa973.
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1-23. doi:10.1007/978-1-4939-2438-7.
- Louie JK, Yagi S, Nelson FA, et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005 Jul 15;41(2):262-5. doi: 10.1086/430915. Epub 2005 Jun 13. PMID: 15983926; PMCID: PMC7107978.
- Outbreaks of human metapneumovirus in two skilled nursing facilities — West Virginia and Idaho, 2011–2012. Centers for Disease Control and Prevention. November 2013. http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html. Accessed September 15, 2020.
- Communicable Disease Service – Adenovirus. State of New Jersey Department of Health. February 2019. https://www.nj.gov/health/cd/topics/adenovirus.shtml. Accessed September 11, 2020.
- CDC expects 2020 outbreak of life-threatening acute flaccid myelitis. Centers for Disease Control and Prevention. August 2020. https://www.cdc.gov/media/releases/2020/p0803-acute-flaccid-myelitis-outbreak.html. Accessed September 15, 2020.
- Waites KB, Xiao L, Liu Y, et al. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev. 2017 Jul;30(3):747-809. doi: 10.1128/CMR.00114-16. PMID: 28539503; PMCID: PMC5475226.
- Kim D, Quinn J, Pinsky B, et al. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085-2086. doi:10.1001/jama.2020.6266.
- Mandelia Y, Procop GW, Richter SS, et al. Dynamics and predisposition of respiratory viral co-infections in children and adults. Clin Microbiol Infect. 2020 Jun 12:S1198-743X(20)30342-6. doi: 10.1016/j.cmi.2020.05.042. Epub ahead of print. PMID: 32540470.
- COVID-19 and antimicrobial resistance (AMR) and pandemics. The Clinical & Laboratory Standards Institute (CLSI). August 2020. https://clsi.org/about/blog/covid-19-and-antimicrobial-resistance-amr-and-pandemics/. Accessed September 15, 2020.
- Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Centers for Disease Control and Prevention. September 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed September 23, 2020.
- Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5(5):401-411. doi:10.1016/S2213-2600(17)30120-0.
- Weiss ZF, Cunha CB, Chambers AB, et al. Opportunities Revealed for Antimicrobial Stewardship and Clinical Practice with Implementation of a Rapid Respiratory Multiplex Assay. J Clin Microbiol. 2019;57(10):e00861-19. Published 2019 Sep 24. doi:10.1128/JCM.00861-19
- Rapid Influenza Diagnostic Tests. Centers for Disease Control and Prevention. October 2016. https://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm. Accessed 9/21/20.
About the Author

Adam Thornberg, MHS
Is Senior Manager of Scientific and Medical Affairs, GenMark Dx