Transfusion-related thrombocytopenia in a chronic renal failure patient
Hemostasis is a process to stop bleeding that requires coordinated activities of vascular, platelet, and plasma factors. Under normal conditions, blood vessel injury will trigger endothelial cells to secrete factors that promote adhesion and activation of platelets. First, platelets bind to von Willebrand’s factor (vWF) secreted by endothelial cells through vWF receptors. Attached platelets then undergo degranulation and release factors such as serotonin, which causes vascular constriction. Activated platelets also release other mediators to attract additional platelets for aggregation at the injured sites. The platelet surface has fibrinogen receptors through which fibrinogens connect adjacent platelets. Platelets will further activate coagulation factors in the plasma and convert prothrombin to thrombin. Thrombin then converts fibrinogen to fibrin, and fibrin strands bind aggregated platelets to form blood clot.1 In this process, platelets play a critical role, and deficiency in platelet count or function may cause uncontrolled bleeding. Thrombocytopenia, a decrease in platelet number, is one of the most common hematological disorders associated with excessive bleeding.
There are two major causes of thrombocytopenia: decreased production and increased breakdown of platelets. Many diseases can lead to a decreased platelet production in the bone marrow. For example, the myelodysplastic syndromes, which are characterized by a defect in hematopoiesis, often cause low platelet counts as well as functionally and morphologically abnormal platelets in the peripheral blood.2 Liver cirrhosis can also cause thrombocytopenia because the liver is the main organ producing thrombopoietin (TPO). TPO stimulates the production and differentiation of megakaryocytes and is required for platelet production.3 Certain drugs such as chemotherapy drugs can cause thrombocytopenia by inhibiting the proliferation of hematopoietic progenitor cells.4
Increased destruction of platelets is another major cause of thrombocytopenia in the clinical setting. Drugs such as heparin, a commonly used anticoagulant, can induce immune response. The resultant antibodies can target platelets for destruction.5 Under certain disease conditions, disseminated intravascular coagulation (DIC) causes blood clot formation throughout the body’s small blood vessels, which will use up platelets and clotting factors in the blood. As a result, DIC is often associated with serious internal and external bleeding.6
Pathogenesis of renal failure
Renal failure is among those conditions that have been associated with thrombocytopenia. Epidemiological studies have shown that both acute and chronic renal failure are associated with anemia and thrombocytopenia.7 Acute renal failure is a rapid decrease in renal function, which leads to a marked increase in serum creatinine and BUN. The inability of the kidney to eliminate waste is associated with a high rate of mortality. Chronic renal failure is a progressive loss of renal function, and patients with chronic renal failure usually end up with hemodialysis. It is known that patients at various stage of chronic renal failure display many abnormalities in hemostasis.8 These patients have increased risks of both thrombotic events and bleeding. Chronic renal failure can also be secondary to other diseases such as type 2 diabetes. With an increasing number of type 2 diabetic patients, it is important to evaluate kidney disease-associated hemostatic disorders. In the following case study, severe thrombocytopenia refractory to transfusion in an end-stage renal failure patient is presented.
Case study presentation on end-stage renal disease
A 65-year-old African American male with end-stage renal disease was admitted to the hospital for a large abdomen mass and bleeding. Four months before, this patient had been found to have a pelvic mass, and the biopsy showed blood and necrotic debris in the mass. The diagnosis at that time was liquefying hematoma. He had returned to the hospital one month before for the accumulation of fluid in the peritoneal cavity (ascites). About 400 ml of cloudy fluid was removed and two different diphtheroids grew from the fluid, suggesting an infection. Consistent with this diagnosis, the patient had 25×103/ml of WBC initially but fell to the normal range quickly after the treatment.
During his admission in the hospital, the patient had several periods of thrombocytopenia, but his platelets were normalized after transfusion, and he was discharged with drains in place. The patient did not have fever, chills, or sweats. He was on Augmentin and receiving hemodialysis. Upon the latest admission, the patient started to bleed again, and his Hgb and platelet levels were critically low (Table 1). Heparin-induced thrombocytopenia (HIT) was suspected and HIT screen was positive, but serotonin release assay was negative. He had been dialyzed without heparin since then. The patient had some bleeding from drain sites, but CT scan did not isolate other sources of bleeding. The amount of bleeding from the drain sites did not seem enough to explain his dropping Hgb and platelet.
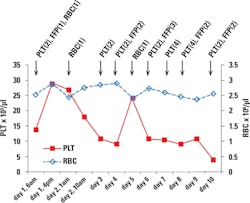
The patient received two units of platelet, one unit of FFP, and one unit of RBC on the first day. The transfusions doubled his platelet count but failed to bring up his RBC (Figure 1), indicating he was still bleeding. DIC was also suspected in the patient. D-dimer value and fibrinogen level were determined on the second day. He had an elevated D-dimer (2.95 mg/ml) but normal fibrinogen (415 mg/dL). His PT and aPTT were elevated slightly, but his INR was normal (Table 1). The patient’s platelet count dropped quickly during the next couple of days.
On day 4, he received two units of platelet and two units of FFP, but that brought his platelet count up only temporarily. His platelet count kept dropping despite daily platelet and FFP transfusion (Figure 1). His CBC profile on day 10 was similar to that on day 1, with an even lower level of platelet (Table 1). The patient developed respiratory distress due to the volume overload, and this eventually led to respiratory arrest. The patient died on day 11.
During this 10-day period, the patient’s RBC and Hgb were low but stable, while his platelet level decreased continuously. Unlike during his previous hospital admissions, this time his thrombocytopenia was refractory to FFP and platelet transfusion.
Analysis of case study
Thrombocytopenia is commonly seen in hemodialysis patients. Since heparin is the most commonly used anticoagulant during dialysis, heparin-induced thrombocytopenia (HIT) is a concern in dialysis patients. It has been shown that one percent to five percent of patients exposed to heparin may develop HIT.9-11 There are two types of HIT: non-pathogenic and pathogenic. Assay for HIT antibodies usually detects both types of HIT. The non-pathogenic HIT does not cause thrombocytopenia despite the presence of HIT antibodies, while pathogenic HIT may have a catastrophic consequence.
Heparin binds to PF4, and the heparin-PF4 complex is immunogenic, which induces the expression of IgG, as well as IgA and IgM antibodies against the heparin-PF4 complex. Although these antibodies could destroy platelets and lead to thrombocytopenia, HIT is rarely associated with bleeding.5 Consistent with this notion, the patient in this case had a positive HIT antibody result and low count of platelet, but his bleeding was not prominent. In fact, HIT is often associated with thromboembolic manifestations including venous thrombosis and myocardial infarction.5 This is due to the fact that heparin tends to cause platelet self-aggregation and activation. It has been shown that heparin interacts with integrins on the platelet surface, and this interaction may cause platelet self-aggregation.12 In addition, heparin can cause platelet degranulation, which activates platelets.13 That may explain HIT-associated clotting of extracorporeal circuit in dialysis patients.14 It is not clear whether this patient developed thrombosis; however, there was no report of clotting in his dialysis tubes.
In this case, HIT alone may not be sufficient to explain the critical low platelet count. It has been reported that the mean decrease of platelet count in HIT patients is about 12 percent.15 This patient had a very low level of platelet even after he had been dialyzed without heparin. Thrombocytopenia has also been linked to biocompatible membranes used in hemodialysis. In hemodialysis, the activation of complement can lead to thrombocytopenia,16 and cellulose membrane has been shown to activate complement.17 Furthermore, electron beam sterilization of polysulfone dialysis membrane has also been linked to thrombocytopenia.18 Although it is unclear what type of dialysis membrane and sterilization method was used in this patient, it is possible that dialysis equipment might have contributed to the patient’s decreased platelet count.
Besides the external factors that cause thrombocytopenia, the patient’s physical condition is probably the most important factor. Anemia and thrombocytopenia have been found in patients with acute and chronic renal failure.7,19 Red cell production is stimulated by erythropoietin (EPO), and the kidney is the source to secrete EPO. In patients with chronic renal failure, deficiency in EPO production is the main cause of the development of anemia. Anemia in this patient might be a combined consequence of EPO deficiency and bleeding. For platelet production, its stimulator, TPO, is constitutively produced by the liver but is also produced by the kidney.20 Therefore, it would be reasonable to expect TPO deficiency in chronic renal failure patients. However, it seems that TPO deficiency due to the kidney failure is negligible as a cause of the thrombocytopenia.21 In fact, serum TPO level is elevated in dialysis patients.21 Nevertheless, megakaryocytes are reduced in the bone marrow of renal failure patients, indicating a deficiency in platelet production, although the molecular mechanisms are not clear.
The platelet function in chronic renal failure patients is also jeopardized. Under normal conditions, ADP and serotonin are secreted to attract more platelets. In renal failure patients, their platelet granules have decreased levels of ADP and serotonin.22 Anemia could further worsen the bleeding disorder because red cells release ADP and facilitate platelets contacting with subendothelium at the damage site.23
Overall, in chronic renal failure patients both platelet count and function are decreased. What was unique in this case was that the thrombocytopenia was refractory to massive platelet transfusion. If this was due to the nonstop bleeding, then one would expect the patient’s RBC and Hgb to decrease dramatically as well. In fact, his CBC profiles showed that other cell counts were pretty stable, and no massive bleeding was noticed. These results suggested that transfused platelets were either destroyed or consumed rapidly. DIC is a common cause of the exhaustion of coagulation factors and platelets, thereby causing thrombocytopenia and bleeding. DIC was suspected in this patient.
However, the diagnosis of DIC remains difficult. An elevated level of D-dimer is one of the diagnostic elements for DIC. The patient’s D-dimer was above the normal range when he was admitted to the hospital. However, D-dimer alone may not be sufficient for DIC diagnosis. A study has shown that 70 percent to 90 percent of hospitalized patients without DIC also have D-dimer values greater than the upper limit of the reference interval.24 That study suggests a cutoff of 8.2mg/ml to “rule in” a diagnosis of DIC.24 The patient’s D-dimer level in this case study was much lower than this cutoff value, suggesting DIC might not be a major concern for the patient. Since coagulation factors are depleted in DIC, prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT) are often associated with DIC. A prolonged PT or aPTT has been observed in 95 percent of patients with DIC.25,26 The patient in this case study had slightly prolonged PT (15.2 sec) and aPTT (36.2 sec) but normal INR (1.2). In addition, his fibrinogen was in normal range. Again, those results suggested that DIC might not be a major complication in this patient. In summary, thrombocytopenia in this patient was likely a combined consequence of platelet consumption and destruction, as well as bleeding.
Conclusion
Patients with chronic renal failure could develop severe thrombocytopenia and platelet dysfunction as well as bleeding. Furthermore, these conditions could be refractory to transfusion. Mechanistic research, epidemiological study, and case report on renal disease-caused hemostatic disorders may help us understand the underlying mechanisms and develop treatment strategies.
REFERENCES
- McKenzie SB. Clinical Laboratory Hematology 2nd Ed. New York: Pearson, Inc. 2009.
- Foran JM, Shammo JM. Clinical presentation, diagnosis, and prognosis of myelodysplastic syndromes. Am J Med. 2012;125:S6-13.
- Giannini EG, Savarino V. Thrombocytopenia in liver disease. Curr Opin Hematol. 2008;15:473-480.
- Bodensteiner DC, Doolittle GC. Adverse haematological complications of anticancer drugs. Clinical presentation, management and avoidance. Drug Saf. 1993;8(3):213-224.
- Lovecchio F. Heparin-induced thrombocytopenia. Clin Toxicol (Phila). 2014;52(6):579-583.
- Hossain N, Paidas MJ. Disseminated intravascular coagulation. Semin Perinatol. 2013;37(4):257-266.
- Dorgalaleh A, Mahmudi M, Tabibian S, et al. Anemia and thrombocytopenia in acute and chronic renal failure. Int J Hematol Oncol Stem Cell Res. 2013;7(4):34-39.
- Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010;36(1):34-40.
- Asmis LM, Segal JB, Plantinga LC, et al. Heparin-induced antibodies and cardiovascular risk in patients on dialysis. Thromb Haemost. 2008;100(3):498-504.
- Yamamoto S, Koide M, Matsuo M, et al. Heparin-induced thrombocytopenia in hemodialysis patients. Am J Kidney Dis. 1996;28(1):82-85.
- Matsuo T, Kobayashi H, Matsuo M, et al. Frequency of anti-heparin-PF4 complex antibodies (HIT antibodies) in uremic patients on chronic intermittent hemodialysis. Pathophysiol Haemost Thromb. 2006;35(60:445-450.
- Sobel M, Fish WR, Toma N, et al. Heparin modulates integrin function in human platelets. J Vasc Surg. 2001;33(3):587-594.
- Gritters M, Borgdorff P, Grooteman MP, et al. Platelet activation in clinical haemodialysis: LMWH as a major contributor to bio-incompatibility? Nephrol Dial Transplant. 2008;23(9):2911-2917.
- Syed S, Reilly RF. Heparin-induced thrombocytopenia: a renal perspective. Nat Rev Nephrol. 2009;5(9):501-511.
- Luzzatto G, Bertoli M, Cella G, Fabris F, Zaia B, Girolami A. Platelet count, anti-heparin/platelet factor 4 antibodies and tissue factor pathway inhibitor plasma antigen level in chronic dialysis. Thromb Res. 1998;89(3):115-122.
- Hakim RM, Schafer AI. Hemodialysis-associated platelet activation and thrombocytopenia. Am J Med.1985;78(4):575-580.
- Muir KB, Packer CD. Thrombocytopenia in the setting of hemodialysis using biocompatible membranes. Case Rep Med. 2012;2012:358024.
- Kiaii M, Djurdjev O, Farah M, Levin A, Jung B, MacRae J. Use of electron-beam sterilized hemodialysis membranes and risk of thrombocytopenia. JAMA. 2011;306(15):1679-1687.
- Gafter U, Bessler H, Malachi T, Zevin D, Djaldetti M, Levi J. Platelet count and thrombopoietic activity in patients with chronic renal failure. Nephron. 1987;45(3):207-210.
- Sungaran R, Markovic B, Chong BH. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood. 1997;89(1):101-107.
- Ando M, Iwamoto Y, Suda A, Tsuchiya K, Nihei H. New insights into the thrombopoietic status of patients on dialysis through the evaluation of megakaryocytopoiesis in bone marrow and of endogenous thrombopoietin levels. Blood. 2001;97(4):915-921.
- Di Minno G, Martinez J, McKean ML, De La Rosa J, Burke JF, Murphy S. Platelet dysfunction in uremia. Multifaceted defect partially corrected by dialysis. Am J Med. 1985;79(5):552-559.
- Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19(4):317-322.
- Lehman CM, Wilson LW, Rodgers GM. Analytic validation and clinical evaluation of the STA LIATEST immunoturbidimetric D-dimer assay for the diagnosis of disseminated intravascular coagulation. Am J Clin Pathol.2004;122(2):178-184.
- Chakraverty R, Davidson S, Peggs K, Stross P, Garrard C, Littlewood TJ. The incidence and cause of coagulopathies in an intensive care population. Br J Haematol. 1996;93(2):460-463.
- MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39-44.
Xiaoming Yang, PhD, MLS(ASCP), serves as a medical laboratory scientist at Dorn VA Medical Center in Columbia, SC.
Floyd Josephat, EdD, MT(ASCP), is a faculty member in the Clinical Laboratory Science Program, University of Alabama at Birmingham.