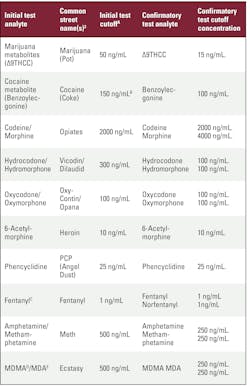
Cutoff Concentrations for Drug Tests
To download Cutoff Concentrations for Drug Tests, click here.
This table was put together by MLO staff and reviewed by Suhash Harwani, PhD, Senior Director of Science for Workforce Health Solutions at Quest Diagnostics. The table refers to federal regulations at 42 CFR Chapter 1, HHS Drug Testing Panel—Urine and 49 CFR part 40, Section 40.85. New to the table this year is fentanyl and norfentanyl due to the prevalence of fentanyl use and overdoses.
The table follows:1,2
References
1. Mandatory guidelines for Federal workplace drug testing programs-authorized testing panels. Federal Register. January 16, 2025. Accessed June 6, 2025. https://www.federalregister.gov/d/2025-00425.
2. 49 CFR part 40 -- procedures for transportation workplace drug and alcohol testing programs. Ecfr.gov. Accessed June 17, 2025. https://www.ecfr.gov/current/ title-49/subtitle-A/part-40.
3. SAMHSA guidelines - US. Accessed May 2, 2025. https://www.thermofisher.com/us/en/home/clinical/diagnostic-testing/clinical-chemistry-drug-toxicology-testing/drugs-abuse-testing/drug-testing-overview/samhsa.html.
A. For grouped analytes (i.e., two or more analytes that are in the same drug class and have the same initial test cutoff):
Immunoassay: The test must be calibrated with one analyte from the group identified as the target analyte. The cross-reactivity of the immunoassay to the other analyte(s) within the group must be 80 percent or greater; if not, separate immunoassays must be used for the analytes within the group.
Alternate technology: Either one analyte or all analytes from the group must be used for calibration, depending on the technology. For a technology that measures a response from the entire group without differentiating between analytes (e.g., an activity-based assay, a mass spectrometric assay that does not differentiate isobaric compounds), the laboratory must compare the result to the initial test cutoff. In the case of an alternate technology that differentiates and quantifies each analyte in the group, the laboratory must compare each analyte’s result to the confirmatory test cutoff and reflex specimens with a positive initial test result to confirmatory testing.
B. Alternate technology (BZE): The confirmatory test cutoff must be used for an alternate technology initial test that is specific for the target analyte (i.e., 100 ng/ mL for benzoylecgonine).
C. A fentanyl immunoassay must have at least 5% cross-reactivity to norfentanyl.
D. Methylenedioxymethamphetamine (MDMA).
E. Methylenedioxyamphetamine (MDA).
About the Author
Suhash Harwani, PhD
is the Senior Director of Science for Workforce Health Solutions at Quest Diagnostics.