Clostridioides difficile in inflammatory bowel disease
The intestinal microbiota in the human large intestine is a highly specialized ecosystem that has evolved over millions of years. Here reside trillions of anaerobic bacteria comprising more than 1,000 species — many more than in the small intestine.
This community maintains a healthy symbiotic relationship with us, with the total number of bacterial cells and genes vastly outnumbering the number of human cells and genes in the body. Our large intestine provides a little over 6 square feet of living space in an oxygen-free environment for the anaerobes while these bacteria supply us with basic physiological and biochemical functions to keep us healthy and safe.
The microbiota is a virtual organ that interacts with other body organs, but much of how this interaction occurs remains poorly understood. We do know, however, that the microbiota provides key functions such as tightly regulated immune homeostasis, metabolic activities such as bile acid metabolism and hormonal balance, and neuronal interaction of the gut-brain axis that involves neurotransmitters and inflammatory signals.
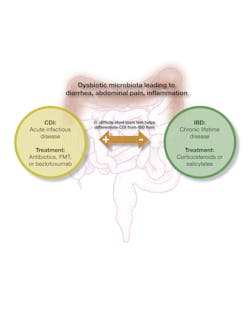
When this diverse ecosystem is disrupted, our health can be endangered. This phenomenon is well illustrated with two diseases, inflammatory bowel disease (IBD) and C. difficile infection (CDI). These diseases have very different etiologies, but both are triggered by a dysbiotic (i.e., imbalanced) intestinal microbiota. It is critical that efforts continue to understand the role of the microbiota in these diseases and others like them because dysbiotic-related diseases are major burdens to healthcare. CDI, for example, is the primary hospital-acquired infection in the U.S. and Europe, resulting in hundreds of thousands of cases and tens of thousands of deaths annually. About 25% of patients experience “recurring” CDI. IBD, which is not an infection but instead a chronic, possibly autoimmune, condition, affects 2-3 million people in the U.S., either as Crohn’s Disease or ulcerative colitis. IBD is becoming a more global disease as countries become more industrialized.
The challenge of diagnosing CDI in an IBD patient
CDI and IBD present with similar clinical features that include diarrhea, abdominal pain/tenderness, and inflammation, but they require very different therapies. CDI, typically triggered by antibiotic therapy, can be effectively treated in most instances with other antibiotics, primarily vancomycin or fidaxomicin. IBD, on the other hand, usually requires corticosteroids for Crohn’s Disease or salicylates for ulcerative colitis. Without an accurate diagnosis, inappropriate treatment with either disease may lead to a life-threatening event. Diagnosing a patient with CDI or an IBD patient who is flaring is challenging enough. But when an IBD patient tests positive for C. difficile, the challenge becomes even greater. Is it CDI or an IBD flare? Recurrent bouts of either disease can further complicate the diagnosis.
Distinct etiologies of CDI and IBD
C. difficile is a prototypical opportunistic pathogen that infects the large intestine. The organism is a gram-positive, anaerobic, spore-forming bacterium that is virulent because of the potent toxins it produces. Fortunately, C. difficile cannot grow in the intestine when there is a healthy, diverse microbiota present. However, when the microbiota is disrupted, most often by antibiotics, the spores which have entered the patient by the fecal-oral route germinate into actively growing vegetative cells. Clearly, it is the disruption of the microbiota that triggers the onset of CDI. The vegetative cells grow to high numbers because the healthy competing microbiota has been killed by the antibiotics, and there is very little competition for nutrients required by C. difficile.1 As this pathogen grows in the large intestine, it produces toxins A and B, both of which are large glucosylating toxins that damage the intestinal mucosa by shutting down the intracellular cytoskeletal system. In addition, the toxins trigger inflammation through several mechanisms that include direct chemotactic activity and cellular damage. CDI can range from mild, self-limiting diarrhea to severe life-threatening colitis.
IBD is a chronic and relapsing intestinal disease, probably autoimmune in origin. The two primary forms of IBD, Crohn’s Disease and ulcerative colitis, have slightly different clinical features. Crohn’s Disease occurs more commonly in younger adults and tends to involve multiple layers of the mucosa anywhere along the intestinal tract. Ulcerative colitis is localized to the colon and rectum, involves more superficial ulceration and inflammation of the mucosa, and attacks a wider age group. There is a genetic predisposition to IBD, and some putative genetic markers have been identified that help to explain why, for example, family members of a Crohn’s patient have a greater likelihood of developing the disease.
IBD patients carry C. difficile at increased rates and are more at-risk for CDI
Persons with IBD have a less diverse intestinal microbiota. There is a reduction in the number of anti-inflammatory members of the microbiota such as the Firmicutes accompanied by higher numbers of pro-inflammatory members such as Bacteroidetes.2 This reduction in diversity is a key characteristic of IBD and represents a triggering event for carriage of C. difficile by IBD patients. Because of the higher carriage, there is an accompanying increased risk of CDI.3
In one study, the prevalence of CDI was 37.3 per 1000 in patients with ulcerative colitis, 10.9 per 1000 in patients with Crohn’s Disease, and 4.5 per 1000 among patients without IBD.4 In the UK, where CDI rates have decreased over the past 10 years, complications of CDI in IBD patients also have decreased.5 In addition to clinical epidemiological data, recent research in mouse models has shown that the onset of IBD is a predisposing factor for both C. difficile colonization and CDI.6 Additional new information indicates that the risk of CDI in IBD patients is increased by factors such as healthcare exposures, nonsteroidal anti-inflammatory drugs, colonic vs intestinal involvement, and whether they are transplant recipients. IBD patients with CDI are at higher risk for complications, IBD flares, colectomies, and death than patients without IBD.7
Inflammatory markers help identify severe IBD and CDI but do not distinguish between the diseases
With some diseases, the presence of inflammation can be a distinguishing clinical feature. IBD, for example, can be differentiated from irritable bowel syndrome, which has overlapping clinical features, but which is noninflammatory. In the case of both CDI and IBD, however, inflammation is a clinical hallmark, and the presence or absence of inflammation is not a differentiating feature. In both diseases, peripheral white blood cell counts and fecal biomarkers of inflammation (e.g., fecal lactoferrin released from infiltrating leukocytes) are elevated. The increase in these inflammatory markers during CDI, more so than the fecal bacterial burden, correlates with severity.8
With CDI, there is accompanying involvement of inflammasomes that play a role in proinflammatory cytokines, but it is unclear whether CDI-associated cytokines may serve as surrogate markers for the disease. With IBD, neutrophil-to-lymphocyte ratios have been examined as a means of establishing a diagnosis of IBD along with severity.9 However, there is no clear indication that this approach may help differentiate CDI from an IBD flare.
Laboratory tests help distinguish CDI from IBD
Widely used tests for C. difficile and its disease include PCR and glutamate dehydrogenase (GDH), both of which detect the organism, and stool toxin, which detects toxins A and B. PCR is very sensitive, but the high sensitivity results in lower positive predictive values because C. difficile carriage is common. GDH can detect actively growing bacteria, but the test does not differentiate between toxigenic and nontoxigenic strains, which do not cause disease. For CDI, patient clinical history in conjunction with toxin detection offers higher positive predictive values than either PCR or GDH testing. Microbiology and gastroenterology societies now recommend an algorithm approach that consists of PCR or GDH screen coupled with toxin testing for accurate diagnosis of CDI.
These recommendations have been extended to aid in the diagnosis of CDI in IBD patients. Recent guidelines and recommendations report that the detection of stool toxin helps to accurately diagnose CDI in IBD patients.10,11 Further, the recommendations state that IBD patients who flare should be tested for CDI and for recurrent CDI if the diarrhea and colitis symptoms persist. A negative PCR or GDH result can help rule out CDI in an IBD patient.12,13 If the toxin test is positive, treatment with vancomycin or fidaxomicin should be considered. If the patient’s condition does not improve within several days, then additional clinical workup for an IBD flare should be considered.
Fecal microbiota transplantation (FMT) can restore homeostasis
Efforts to restore the intestinal microbiota in patients with CDI and IBD through fecal microbiota transplantation (FMT) have met with varying levels of success. In patients with recurrent CDI, FMT has a high success rate. Multiple companies are actively researching ways to improve the technology of FMT. The approaches range from using fecal emulsions obtained from donors who have been rigorously tested for the absence of C. difficile and other pathogens to efforts that consist of well-defined bacterial populations delivered in pill form.
The fecal emulsion approach relies on the collection, screening, and shipping of fecal specimens and the preparation of samples for instillation in patients. The procedure has reported success rates >90%. Clinical studies on more defined combinations of bacteria that are curative and that prevent recurrent CDI are promising. With treatment of IBD, FMT results have not been as spectacular. Even so, clinical research still is underway on the utility of FMT as a treatment in IBD patients.14 IBD may be less responsive to FMT and more challenging than CDI because the goal is to establish a more diverse microbiota than what currently exists in an IBD patient rather than simply replacing the microbiota, the goal in a CDI patient. FMT has been recommended as a treatment for IBD patients who are toxin-positive with recurrent CDI.11,15
Conclusions
IBD and CDI are intestinal diseases that present with similar clinical features but have very different etiologies. Both are the result of a dysbiotic intestinal microbiota. In CDI, the microbiota becomes dysbiotic after antibiotics or other medications/conditions that disrupt the microbiota, setting up the possibility of disease. In IBD, the cause of the dysbiosis is unclear, but significant disruption of the microbiota is evident. As a result, IBD patients have a higher likelihood of being colonized with C. difficile and developing CDI. Because of the similar clinical features and the higher rates of carriers and CDI in IBD patients, it can be difficult to distinguish a true CDI infection from an IBD flare. Recent recommendations include the detection of C. difficile toxin as an aid in the diagnosis of CDI in IBD patients.
Jodie Y. Lee, MS, MBA, serves as Marketing Manager for TECHLAB. She has spent 16 years in the life science and diagnostic industries.
David M. Lyerly, PhD, is a Co-Founder and serves as Chief Science Officer for TECHLAB, Blacksburg, VA, which manufactures a variety of in vitro diagnostic tests for enteric diseases.
References
- Jenoir M, Leslie J, Young V, Schloss P. 2017. Clostridium difficile colonizes alternative nutrient niches during infection across distinct murine gut microbiomes. mSystems 2:e00063-17. doi: org/10.1128/mSystems.00063-17.
- Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol 2015;50:495-507. doi: 10.1007/s00535-015-1064-1.
- Rao K, Higgins P. Epidemiology, diagnosis, and management of Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis 2016;22:1744-1754. doi: 10.1097/MIB.0000000000000793.
- Nguyen G, Kaplan G, Harris M, et al. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol 2008;103:1443-1450.
- Joshi N, Marks I, Crowson R, et al. Incidence and outcome of Clostridium difficile infection in hospitalized patients with inflammatory bowel disease in the UK. J Crohn’s and Colitis 2017;70-76. doi: 10.1093/ecco-jcc/jjw117.
- Abernathy-Close L, Barron M, George J, et al. Intestinal inflammation and altered gut microbiota associated with inflammatory bowel disease render mice susceptible to Clostridioides difficile colonization and infection. mBio 12:e:02733-20. doi: org/10.1128/mBio.02733-20.
- Lam A, Gutin L, Nguyen Y, et al. Management of recurrent Clostridioides infection: a difficile problem in inflammatory bowel disease patients. Digest Dis Sci 2020;65:3111-3115. doi: org/10.1007/s10620-020-06521-x.
- El Feghaly R, Stauber J, Deych E, et al. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin Infect Dis. 2013;56:1713-1721. doi: 10.1093/cid/cit147.
- Langley B, Guedry S, Goldenberg J, et al. Inflammatory bowel disease and neutrophil-lymphocyte ratio: a systematic scoping review. J Clin Med 2021:10:4219-4244. doi: org/10.3390/jcm10184219.
- Khanna S, Shin A, Kelly C. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the clinical practice updates committee of the AGA institute. Clin Gastroenterol Hepatol 2017;15:166-174.
- Khanna S. Management of Clostridioides difficile infection in patients with inflammatory bowel disease. Intest Res 2021;19:265-274. doi: org/10.5217/ir.2020.00045.
- Allegretti J. Clostridioides difficile infection in IBD: making the appropriate diagnosis. https://www.youtube.com/watch?v=xkYl3rMzKHM.
- Dalal R, Allegretti J. Diagnosis and management of Clostridioides difficile infection in patients with inflammatory bowel disease. Curr Opin Gastroenterol 2021:37:336-343. doi: 10.1097/MOG.0000000000000739.
- Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 2018;11:1-10. doi: org/10.1007/s12328-017-0813-5.
- Allegretti J, Kelly C, Grinspan A, et al. Inflammatory bowel disease outcomes following fecal microbiota transplantation for recurrent C. difficile infection. Inflamm Bowel Dis 2021;27:1371-1378. doi: 10.1093/ibd/izaa283.
About the Author

Jodie Y. Lee, MS, MBA
serves as Marketing Manager for TECHLAB. She has spent 16 years in the life science and diagnostic industries.

David M. Lyerly, PhD
is a Co-Founder and serves as Chief Science Officer for TECHLAB, Blacksburg, VA, which manufactures a variety of in vitro diagnostic tests for enteric diseases.