The role of intestinal inflammation in Clostridioides difficile disease
In 2019, there were more than 250,000 cases of Clostridioides difficile infection (CDI) in the United States, possibly as many as 500,000, resulting in the death of up to 30,000 patients. Healthcare costs were well in excess of a billion dollars. Figures in Europe were staggering as well, and the numbers of cases of CDI reported elsewhere around the world increased. CDI leapfrogged over Methicillin-resistant (MRSA) infections years ago as the most common hospital-acquired infection (HAI) in the United States.
C. difficile is a prototypical opportunistic pathogen
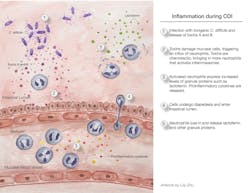
C. difficile is very opportunistic. It is capable of taking advantage of a weakened intestinal microbiota, and it forms very hardy endospores. Consequently, CDI’s continuing presence at the top of the HAI list is no surprise. A single CDI patient sheds millions of endospores daily. Endospores are spread in healthcare facilities even when mitigation efforts are in place. Unfortunately, the endospores are often shed where there is a highly susceptible population, such as hospitalized patients who have a weakened microbiota, because they are receiving antibiotics. Endospores enter patients via the fecal-oral route, survive stomach acid, and arrive in the anaerobic environment of the colon where they germinate. In the absence of a diverse healthy microbiota, C. difficile can grow to high numbers. Under these conditions, this pathogenic anaerobe produces deadly toxins A and B that damage the gut mucosa and trigger inflammation.
Our healthy microbiota does an amazing job keeping this pathogen in check, making it difficult to detect C. difficile in healthy adults. Infants, however, are a different story. More than 50 percent of infants carry C. difficile as part of their normal flora, until their "adult" flora develops between 1 and 2 years of age. Why infants have this protection remains a mystery; although, the lack of suitable cellular toxin receptors may be part of the reason. Many patients become carriers of C. difficile, probably because they are exposed to high numbers of endospores while hospitalized and immunocompromised. Carriers in hospitals tend to outnumber true cases of CDI. As a result, it can be difficult to distinguish C. difficile carriers from patients with CDI. Algorithms (e.g., GDH plus toxin, NAAT plus toxin) improve diagnostic accuracy and reduce inappropriate treatment of carriers.
In some patients, CDI is mild, and the disease resolves when an inciting antibiotic is discontinued. Occasionally, patients may have a norovirus or Campylobacter infection, with C. difficile simply being a bystander organism. Infectious diseases are also not the only cause of diarrhea, particularly in hospital settings. In these situations, C. difficile may be blamed for diarrhea with an entirely different etiology. Although efforts should be made to minimize inappropriate treatment of carriers, at the same time, it is critical that patients be accurately diagnosed and treated, because CDI can rapidly progress to colitis and become life-threatening. Up to 25 percent of CDI patients will relapse with recurrent CDI, because the endospores are not killed by the antibiotics used to treat CDI and will linger in the patient. Recurrence can occur multiple times weeks or months apart, each time causing further deterioration of the patient’s health.
Inflammation is a hallmark of CDI
Higher levels of inflammation in CDI are associated with increasingly severe disease. Pseudomembranous colitis (PMC), the severe stage of CDI, is an acute inflammation of the colon with pseudomembranes comprised of necrotic debris and inflammatory cells. Pseudomembranes in the large intestine are clinically diagnostic. Fortunately, with today’s improved laboratory tests and increased awareness of CDI, most patients do not progress to this stage. Peripheral white blood cells (WBC) counts have been used for many years to monitor severity. Counts >15,000 per mm3 signal severe CDI.1 Elevated white cell counts are included as an indicator in the Hines VA Severity Score, an often-used assessment based on fever, ileus, hypotension, white blood cell count, and thickening/dilation of the colonic wall.2
More recently, fecal lactoferrin, an 80 kDa glycoprotein released from secondary granules during degranulation and lysis of fecal leukocytes, has been used to assess inflammation and severity. Fecal lactoferrin is highly stable in feces, can be measured qualitatively and quantitatively, and serves as an accurate biomarker for direct assessment of intestinal inflammation.
Numerous studies have evaluated fecal lactoferrin, peripheral WBC counts, and stool toxin as biomarkers in patients with CDI, and all continue to illustrate the important role of inflammation in CDI. In one study, the correlation of these biomarkers with clinical assessment of severe CDI was examined in patients infected with ribotype 027.3 Ribotype 027 is notorious for causing severe disease. It is a fluoroquinolone-resistant 027 variant that appeared in the early 2000s, causing severe outbreaks in Europe, Canada, and the United States. In some outbreaks, the mortality rates more than doubled in patients with 027-associated CDI. Fortunately, the incidence of 027 infections is decreasing in the United States and in Europe, and this ribotype is not prevalent in Asia. Treatment for an 027 infection is not different from non-027 CDI.
In the 027-based study above, fecal lactoferrin levels >900 µg/g feces were observed in 027-infected patients, highly elevated above the lactoferrin baseline of 7 µg/g. Peripheral WBC counts in this group of patients approached 19,000 per mm3. Patients with moderate CDI had mean fecal lactoferrin levels of 292 µg/g, with mean WBC counts of 13,000 per mm3. Patients with mild CDI had mean fecal lactoferrin levels of 73 µg/g and normal WBC counts of about 9,000 per mm3 .
Another study also assessed 027-infected patients; in this case, patients were admitted to hospitals from long-term care facilities.4 The study similarly showed that 027-infected patients had higher fecal lactoferrin and peripheral WBC counts than non-027-infected patients, and that higher responses with either fecal lactoferrin or peripheral counts correlated with higher mortality rates. Although not focused on ribotype 027, additional studies have demonstrated the correlation of toxin levels with elevated fecal lactoferrin, elevated peripheral WBC counts, and inflammation.5,6
There is supporting evidence that inflammation, determined by elevated fecal lactoferrin or peripheral WBC counts, rather than fecal bacterial burden, correlates with severity.7 This is noteworthy, because it suggests that sustained host inflammatory responses are involved in patients who suffer from prolonged CDI. Further, there is the possibility that prolonged inflammation may be predictive of patients at-risk for relapsing CDI.
Inflammasomes and cytokines play a role in CDI
Inflammasomes are complexes comprised of multiple proteins that become activated and undergo oligomerization. They are associated with inflammatory responses in chronic diseases (e.g., diabetes or inflammatory bowel disease) and infectious diseases (e.g., respiratory diseases, such as COVID, and intestinal infections). There are different types of inflammasomes, depending on which proteins are activated to start the process towards complex oligomerization. In CDI, inflammasome formation appears to be triggered following toxin-mediated cellular damage. The formation of inflammasomes results in activation of caspase 1, with subsequent activation of pro-inflammatory cytokines, such as IL-1β, according to results seen in mouse models of CDI. There possibly are other mechanisms of inflammation, one being pyroptotic cell death defined by cellular death via an inflammatory pathway.
The involvement of inflammasomes in CDI likely serves as an initiating step, based on studies showing that proinflammatory cytokines activated through inflammasomes are upregulated in patients with CDI.8-10 Higher levels of innate host response interleukins in CDI patients are associated with poor prognosis, another signal for inflammation involvement.9 Because inflammasomes are an innate host defense mechanism, cellular signaling appears to be instrumental in determining which interleukins participate in this defense mechanism.
Toxins A and B are key to disease. They not only directly cause tissue damage; they are inflammatory triggers. Both toxins are glucosyltransferases, and this unusual enzymatic activity inactivates G proteins, leading to cell death. When their glucosyltransferase activity is shut down, upregulation of pro-inflammatory cytokines does not occur, strongly supporting the important role of toxin-initiated events. Additional evidence for toxin involvement is provided by in vitro results, showing that treatment of peripheral blood mononuclear cells with toxin leads to responses that are characteristic of inflammasome formation and stimulation of cytokine expression.
Understanding the roles of different cytokines that become activated through this series of events is challenging, but inflammasome-activated secretion of IL-1 seems to be an important first step. The production of IL-1 results in the subsequent production of IL-23, an inflammatory cytokine that signals the involvement of other cytokines.11 Mouse models show that when IL-23 is controlled, CDI is less severe. Looking at the results to-date collectively, this very complex signaling of the inflammatory cascade is instrumental in the upregulation of multiple pro-inflammatory cytokines.
Inflammation plays an important role in CDI
The onset of inflammation in CDI is illustrated in Figure 1. When inflammation is elevated, as determined by clinical assessment, peripheral WBC counts, fecal lactoferrin, or a combination of these, CDI becomes more severe, and the patient’s condition worsens. One episode of CDI is bad enough, but when complicated by multiple relapses exacerbated by inflammation, the patient’s health deteriorates rapidly. Progress is being made in understanding the role of inflammation in CDI and how this process might provide competitive advantages for the organism. For example, inflammation may increase the availability of nutrients to C. difficile, while suppressing the competing microbiota.12
New findings will lead to a more complete understanding of how inflammation is triggered in CDI. Inflammasomes and pro-inflammatory cytokines are involved following complex cellular signaling, indicating that inflammatory cascades are key to this understanding. Hopefully, controlling the inflammation will reduce severity, lower rates of recurrent episodes, and lead to new strategies to treat this disease.
References
- Cohen S, Gerding D, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update for the Society for Healthcare Epidemiology of American (SHEA) and The Infectious Diseases Society of American (ISDA). Infect Control Hosp Epidemiol. 2010;31:431-455. doi: 10.1086/651706.
- Belmares J, Gerding D, Parada J, et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55:495-501. doi: 10.1016/j.jinf.2007.09.015.
- Boone J, DiPersio J, Tan M, et al. Elevated lactoferrin is associated with moderate to severe Clostridium difficile disease, stool toxin, and 027 infection. Eur J Clin Microbiol Infect Dis. 2013;32:1517-1523. doi: 10.1007/s10096-013-1905-x.
- Archbald-Pannone L, Boone J, Carman R, et al. Clostridium difficile ribotype 027 is most prevalent among inpatients admitted from long-term care facilities. J Hosp Infect. 2014;88:218-221. doi: org/10.1016/j.jhin.2014.06.016.
- LaSala P, Ekhmimi T, Hill A, et al. Quantitative fecal lactoferrin in toxin-positive and toxin-negative Clostridium difficile specimens. J Clin Microbiol. 2013;51:311-313. doi: 10.1128/JCM.02735-12.
- Barbut F, Gouot C, Lapidus N, et al. Faecal lactoferrin and calprotectin in patients with Clostridium difficile infection: a case-control study. Eur J Clin Microbiol Infect Dis. 2017;36:2423-2430. doi: 10.1007/s10096-017-3080-y.
- El Feghaly R, Stauber J, Deych E, et al. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin Infect Dis. 2013; 56:1713-1721. doi: 10.1093/cid/cit147.
- Ng J, Hirota S, Gross O, et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 2010;139:542-552. doi: 10.1053/j.gastro.2010.04.005.
- Yu H, Chen K, Sun Y, et al. Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clin Vacc Immunol. 2017;24:e00037-17. doi: org/10.1128/CVI.00037-17.
- Rao K, Erb-Downward J, Walk S, et al. The systemic inflammatory response to Clostridium difficile infection. PLOS One. 2014;9(3);e92578. doi: 10.1371/journal.pone.0092578.
- Cowardin CA, Kuehne SA, Buonomo EL, et al. Inflammasome activation contributes to interleukin-23 production in response to Clostridium difficile. mBio 2015;6(1):e02386-14. doi: 10.1128/mBio.02386-14.
- Fletcher JR, Pike CM, Parsons RJ, et al. Clostridiodes difficile exploits toxin-mediated inflammation to alter the host nutritional landscape and exclude competitors from the gut microbiota. Nature Comm. 2021;12:462. oi: org/10.1038/s41467-020-20746-4.
About the Author

David M. Lyerly, PhD
is a Co-Founder and serves as Chief Science Officer for TECHLAB, Blacksburg, VA, which manufactures a variety of in vitro diagnostic tests for enteric diseases.

Jodie Y. Lee, MS, MBA
serves as Marketing Manager for TECHLAB. She has spent 16 years in the life science and diagnostic industries.