Diamond-Blackfan anemia: a familial case with emphasis on multi-diagnostic approach to diagnosis and treatment
Diamond-Blackfan anemia (DBA) is an autosomal dominant disorder of the bone marrow where an insufficient amount of red blood cells are produced leading to anemia. The condition is named after the pediatricians Louis K. Diamond and Kenneth Blackfan, who described congenital hypoplastic anemia in 1938. This type of anemia usually presents within the first year of life and can lead to a multitude of secondary conditions which will be discussed within this case study.¹ DBA is rare and affects five to seven live births per million worldwide. Corticosteroids is the initial treatment but the Diamond Blackfan Anemia Registry found that 36 percent of those on steroids also require monthly blood transfusions.² This case study will explore a multi-diagnostic approach to testing and treating a familial case of DBA, including hematology, chemistry, and immunohematology. Secondary disorders and diseases such as myelodysplastic syndrome, hemochromatosis, and organ failure will be discussed, as well as gene therapy, bone marrow transplants, and leucine therapy treatments.
Anemia
According to the National Heart, Lung and Blood Institute (2012), anemia is any condition leading to a shortage of red blood cells.³ There are different types of anemia, some not life threatening and some that are very serious. DBA is a type of serious anemia that is chronic and has a strong genetic component. DBA is a failure of the bone marrow to produce an adequate supply of red blood cells. This shortage causes the anemic state in patients and leads to an overall depletion of oxygen throughout the body.4 There are some physical abnormalities that are sometimes associated with this disorder but will not be discussed here, since the patients involved did not demonstrate any such abnormality. Several mutations of ribosomal proteins have been found in those suffering from DBA with 25 percent showing a mutation on the RPS19 gene. This was surprising since the mutation was thought to be due to a deregulation in erythropoiesis.6 This disorder is also autosomal dominant which means that only one copy of the gene is needed to cause the disease.4
Diagnostic criteria
Diagnostic criteria for DBA from the 2008 International Clinical Care Consensus Document are: age less than one year, macrocytic anemia with no other cytopenias, reticulocytopenia, normal bone marrow cellularity with a decrease in red cell precursors, and normal platelet and neutrophil counts.”2 Treatment options for those suffering from DBA initially start with corticosteroid treatment. A large dose is given for the first two weeks after diagnosis to initiate the bone marrow to produce more red blood cells though the mechanism behind this is unknown. After the initial dose, a sustainable dose is given and the possible adverse side effects that come with steroid use are monitored (upset stomach, increased blood sugar, increased blood pressure, and increased risk for infection). The Diamond Blackfan Anemia Registry found that 82 percent were initially responsive to steroids. Thirty-six percent of that 82 percent also received monthly blood transfusions.2
Most patients suffering from DBA have to receive blood transfusions every three to six weeks. The patients hemoglobin is monitored (usually monthly) to ensure it is not dropping to a dangerous level. People with DBA usually have scheduled transfusions to ensure this doesn’t happen. These patients sometimes present a problem for the healthcare worker since getting good access to a vein may be difficult in someone who receives chronic transfusions. It is also a challenge for the patient to receive appropriate blood when they begin developing antibodies to prior blood that they have received. Chronic transfusions are also dangerous since they increase the risk of a transfusion reaction. Those who are transfusion dependent are also at an increased risk of developing a disorder called hemochromatosis, which is essentially iron overload.2
Hemochromatosis is monitored by ferritin levels which indicate the levels of iron in the body. If iron overload is found to be an issue, chelation therapy can be done to remove the excess iron. There are currently two chelation drugs approved by the FDA: Exjade and Desferal. Exjade is an oral drug that binds the iron and removes it through the stool. Desferal is an injectable treatment over eight hours that removes the excess iron through urine.2 Stem cell, or bone marrow transplants are also a possible treatment for those suffering from DBA. If successful, the bone marrow then functions normally and makes an adequate amount of red blood cells. The risks for a bone marrow transplant are numerous, including death if a rejection occurs.2
Laboratory diagnosis
Hematology
Numerous tests performed in the hematology department are vital in a doctor’s treatment of a DBA patient. Those who are transfusionMacrocytosis (red blood cells that are larger than normal) is often seen with DBA. A study done by Pesciotta et al researched whether the macrocytosis was due to protein translation malfunctions by looking at the proteomes in patients with DBA versus samples from healthy patients. Most DBA patients also have an increase in fetal Hgb, a decrease in adenosine deaminase activity, and reticulocytosis. Ribosomal protein S19 accounts for up to 25 percent of the 11 proteins that undergo mutations in DBA. Of the four DBA patients who underwent proteomic analysis, abnormal proteins were found in all four including dysferlin and MHC class 1 proteins. Findings suggest that DBA protein mutations on RBC’s are special unto themselves.7
Incidence of cancer in DBA is highly elevated, more specifically acute myeloid leukemia (AML), and myelodysplastic syndrome (MDS). Clinical presentations and therapies vary greatly between individuals. Vlachos, Atsidatos, Alter, and Lipton did a retrospective study where they took 608 individuals from the DBA registry in North America and measured incidence of MDS and AML. Of the 608 total patients studied, 17 patients with DBA had more than one type of cancer with 15 demonstrating solid tumors and two with AML. Four patients developed MDS. Eight patients were transfusion dependent at the time of cancer diagnosis, two were in remission, and four had never been treated for anemia. Of the 18 who had cancer, ten had a mutation in a ribosomal protein gene known to be associated with DBA. Three of the four most common genotypes were represented: five with RPS19; two with RPL11 (mother and daughter); and two with RPL5. The median overall survival for all patients was 56 years.8
Immunohematology and chemistry
Iron is an important metal within the body responsible for DNA synthesis as well as oxygen binding. There is no method for ridding excess iron naturally so iron overload can be problematic in those receiving multiple transfusions. One unit of red cells has 200 mg of iron, which is more than double the daily recommended dose.10
Ferritin is often used as an indicator for overall iron storage in the body. Generally, ferritin levels are therapeutic in DBA patients at levels between 1,000 and 1,500 ng/mL. These levels are measured every three months and if instability is seen, an MRI is usually ordered to rule out liver and/or tissue damage.5 Liver and cardiac damage is not uncommon in those receiving multiple transfusions since the excess iron leads to deposits (called hemosiderins) accumulating in liver and cardiac tissue.9 Shander, Cappellini, and Goodnough reported on a retrospective study in 2009 that studied 152 patients who had a total of 4,875 units of red cells transfused from 1987-1998. The study found that those who demonstrated iron overload had a much higher organ failure and mortality rate than those who did not show elevated ferritin.10
Treatment
Leucine is an amino acid that aids in protein synthesis regulation and is a possible future treatment for those suffering from transfusion dependent DBA. Jaako et al in 2012 looked at the effects of leucine in a mouse model who was RPS-19 deficient. Double the amount of leucine found in serum was given in drinking water to the mice. The study concluded that of those given the leucine there was a significant increase in red blood cells as well as hemoglobin concentrations. There appeared to be no adverse side effects.11 This has huge implications for those with DBA since the disease carries with it so many adverse secondary complications.
Case study presentation
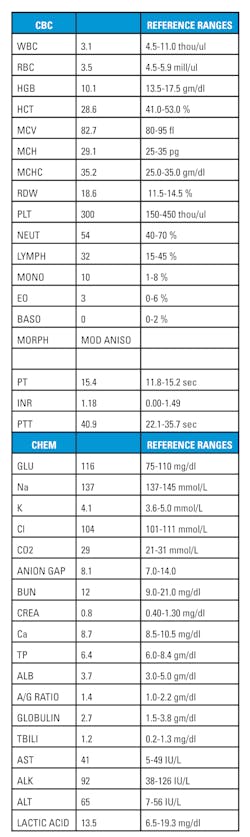
A 29-year-old man presented to the ER with complaints of fever, chills and coughing. His initial exam showed a temperature of 101.1 and a pulse oximetry of 94 percent. During the course of taking the patients history, it was noted that he has DBA and has had iron overload. His current medications included Exajade taken by mouth daily. A PT, PTT/INR, CMP, CBC were ordered, and Ibuprofen was given for the fever. His CBC results showed low WBC, RBC, HGB, HCT, and lymphocyte levels with elevated RDW and neutrophils. His CMP showed high glucose and ALT. (Table 1) His immunohematology records were checked and the patient received a full antigen typing of his red cells, and has since been transfused monthly.
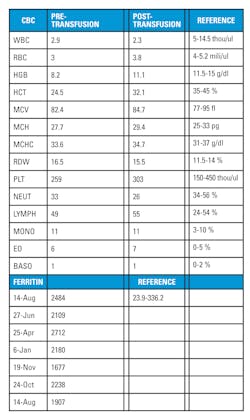
This patient also has two sons, one six and the other five years of age. Both inherited DBA. Their records indicate that they get labs drawn and transfusions on a bi-monthly basis. The oldest son’s labs consistently show low RBC, HGB, HCT, and reticulocyte levels with extremely high ferritin levels. (Table 2) Both children have now started to show signs of autism, but no correlation has been made with autism spectrum disorder and DBA. The children are responding well to the transfusions and no plans have been made for bone marrow transplants.
Conclusion
DBA is a disease of the bone marrow that causes insufficient red cell production. This case study was about a family that suffers from DBA. The case study was developed to provide an overview of the disease with emphasis on a multi-diagnostic approach to help diagnose and treat this disease. DBA presents differently in every person affected and has a variety of treatments. Unfortunately, all of those treatments as well as the disease itself carries many secondary risks.
REFERENCES
- http://www.cdc.gov/ncbddd/dba/facts.html. Published 2019. Accessed May 3, 2019.
- DBAR Findings | Diamond Blackfan Anemia Foundation, Inc. Dbafoundation.org. http://dbafoundation.org/learn-more/dbar-findings/. Published 2019. Accessed May 3, 2019.
- Anemia | National Heart, Lung, and Blood Institute (NHLBI). Nhlbi.nih.gov. http://www.nhlbi.nih.gov/health/health-topics/topics/anemia/. Published 2019. Accessed May 3, 2019.
- Reference G. Diamond-Blackfan anemia. Genetics Home Reference. http://www.ghr.nlm.nih.gov/condition/diamond-blackfan-anemia. Published 2019. Accessed May 3, 2019.
- Vlachos A, Muir E. (2010). How I treat Diamond-Blackfan anemia. Blood, 116(19), 3715-3723. doi:10.1182/blood-2010-02-251090/.
- Flygare J, Karlsson S. (2007). Diamond-Blackfan anemia: erythropoiesis lost in translation. Blood. 109(8), 3152-3154.
- Pesciotta EN, Sriswasdi S, Tang H, Speicher DW, Mason PJ, Bessler M. (2014). Dysferlin and other non-red cell proteins accumulate in the red cell membrane of diamond-blackfan anemia patients. PLoS One, 9(1), e85504. doi:http://dx.doi.org/10.1371/journal.pone.0085504.
- Vlachos A, Rosenberg P, Atsidaftos E, Alter B, Lipton J. (2012). Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood, 119(16), 3815-3819. doi:10.1182/blood-2011-08-375972.
- Stumpf J. (2007). Deferasirox. American Journal of Health-System Pharmacy: AJHP: Official Journal Of The American Society Of Health-System Pharmacists, 64(6), 606-616.
- Shander A, Cappellini MD, Goodnough LT. (2009). Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sanguinis, 97(3), 185-197. doi:10.1111/j.1423-0410.2009.01207.
- Jaako P, Debnath S, Olsson K, Bryder D, Flygare J, Karlsson S. (2012). Dietary L-leucine improves the anemia in a mouse model for Diamond-Blackfan anemia. Blood, 120(11), 2225-2228. doi:10.1182/blood-2012-05-431437.
About the Author

Floyd Josephat, Ed.D., MLS(ASCP)
is currently a professor of hematology and Program Director of the Medical Laboratory Science program at the University of Alabama, Birmingham. Dr. Josephat has been in the medical laboratory science profession for over 30 years and is board certified by the American Society for Clinical Pathology (ASCP) as a medical laboratory scientist.

Katrina Goff
MS, MLS(ASCP), has been a Medical Laboratory Scientist for five years and currently works in the hematology department at the Columbia VA Health Care System in Columbia, SC.